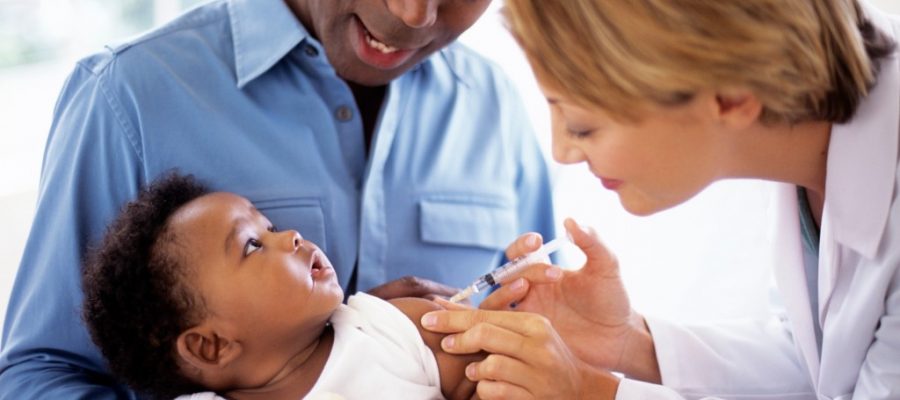
After decades of research, a shot to protect infants from respiratory syncytial virus (RSV) is nearly finalized. However, a semantical snafu could make the treatment inaccessible for low-income families. Here’s everything parents need to know about the forthcoming RSV shot.
As Kaiser Health News reported, the pharmaceutical industry is close to perfecting effective immunizations against RSV, which caused an alarming surge in pediatric hospitalizations throughout the United States last fall. Only one version of the shot, called nirsevimab, is designed for infants. It is intended to be administered before a baby’s first winter RSV season.
Technically, the baby-friend shot is not a standard vaccine. It is a monoclonal antibody treatment, which neutralizes RSV in the bloodstream.
Unfortunately, this small technicality could create financial barriers for families who are uninsured, underinsured, or on Medicaid. All routine pediatric vaccines are available for free to low-income families under the federal Vaccines for Children program, which was implemented in 1994. Since nirsevimab is an antibody treatment, not a vaccine, it would not be covered by the program unless government officials intervene.
In a statement to Kaiser Health News, Kristen Norlund, a spokesperson for the Centers for Disease Control and Prevention (CDC), said the agency is still determining whether nirsevimab would be eligible under the Vaccines for Children program.
Nirsevimab was approved by European officials last December and is expected to be authorized by the Food and Drug Administration (FDA) over the summer. Sanofi and AstraZeneca, the two drug companies responsible for the shot, hope to see it recommended by the CDC and then offered nationwide this fall.
Pfizer and GSK, two other drug companies, are reportedly developing a traditional RSV vaccine. Their shot is designed to be given to pregnant people — not infants themselves — to protect their babies from the virus.
Since both shots are new, they will likely be met with vaccine hesitancy once they become available.
The need for a shot against RSV became more urgent than ever amid this winter’s “tripledemic.” RSV usually peaks in the winter, but cases skyrocketed last fall, causing hospitals to scramble as pediatric beds filled up ahead of schedule. This congestion became even more concerning as cold and flu season ramped up, with cases of the flu and COVID-19 surging concurrently.
Luckily, case numbers for all three viruses appear to be declining, according to The Washington Post.
According to the CDC, RSV is a common virus that spreads via contact with respiratory droplets, an infected person, or a contaminated surface. It manifests as mild, cold-like symptoms for most people. However, RSV can cause serious complications in certain vulnerable populations, including premature infants, infants six months old and younger, children younger than 2 with congenital lung or heart issues, and children with weakened immune systems.
Since RSV affects the respiratory tract, people who develop severe infections may require in-hospital treatment to help them breathe. In most cases, hospitalization only lasts a few days.
Got a sick child at home? Check out these natural products to soothe their cold symptoms:

Source: Read Full Article