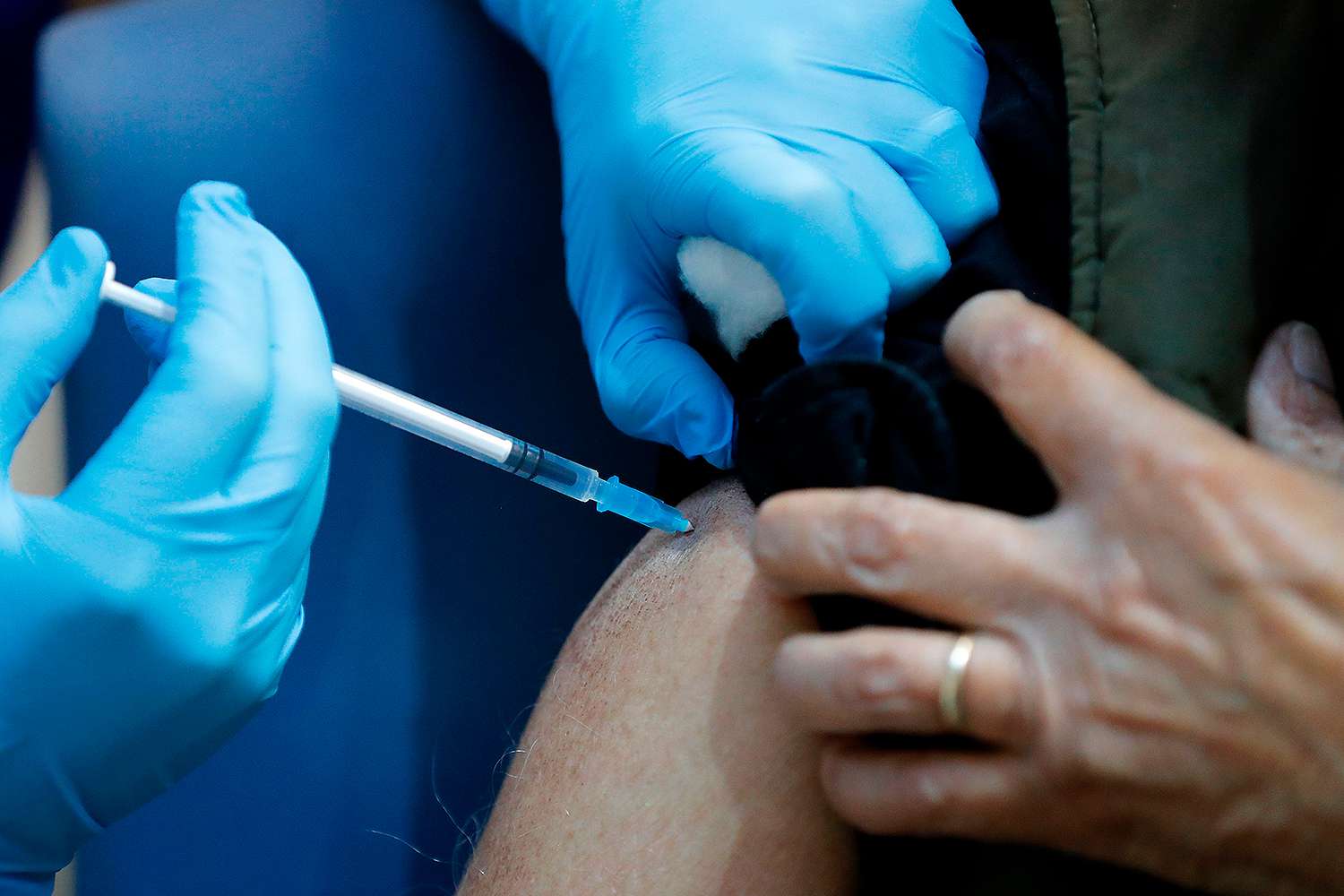
People with a history of severe allergic reactions should not yet get Pfizer’s COVID-19 vaccine, officials in the U.K. told hospitals, after two health care workers had adverse reactions to the shot.
As the U.K. started a mass effort to immunize the country, beginning with health care workers and the elderly, two staffers from Britain’s National Health Service (NHS) with a history of severe allergic reactions developed mild allergy symptoms after getting the vaccine.
Both workers had symptoms of an “anaphylactoid reaction,” which typically includes a skin rash, breathlessness and occasionally a drop in blood pressure, and is milder than the severe anaphylaxis reaction that can be fatal. The health care workers are now fine.
“Both are recovering well,” Stephen Powis, the national medical director for NHS England, said in a statement, CNN reported.
Due to the workers’ reactions, the NHS is currently stopping hospitals from giving Pfizer’s vaccine to people with a “significant history of allergic reactions” while they investigate the issue. Powis said that this is “common with new vaccines.”
"The MHRA [Medicines and Healthcare products Regulatory Agency] have advised on a precautionary basis that people with a significant history of allergic reactions do not receive this vaccination after two people with a history of significant allergic reactions responded adversely yesterday," Powis said.
Pfizer said that they support the MHRA’s investigation. During their clinical trials, 0.6 percent of the 44,000 volunteers had some form of an allergic reaction to the vaccine.
The MHRA recommended that anyone with a history of severe allergic reactions to a vaccine, medicine or food, or those who carry an adrenaline autoinjector pen, should wait to get Pfizer’s vaccine.
"Once all the information has been reviewed we will communicate updated advice," a MHRA spokesperson said, according to CNN.
Pfizer’s vaccine — which was approved for use in Canada on Wednesday — is nearing approval in the U.S. On Tuesday, the Food and Drug Administration said that the vaccine, which requires two shots administered three weeks apart, is more than 50 percent effective after just the first dose, and nearly 100 percent effective after the second.
The FDA is expected to approve Pfizer’s vaccine on Thursday, and states will soon begin distributing it to health care workers and residents of long-term nursing facilities. A second vaccine, from Moderna, is also on track for FDA emergency use approval from the FDA.
As information about the coronavirus pandemic rapidly changes, PEOPLE is committed to providing the most recent data in our coverage. Some of the information in this story may have changed after publication. For the latest on COVID-19, readers are encouraged to use online resources from CDC, WHO, and local public health departments. PEOPLE has partnered with GoFundMe to raise money for the COVID-19 Relief Fund, a GoFundMe.org fundraiser to support everything from frontline responders to families in need, as well as organizations helping communities. For more information or to donate, click here.
Source: Read Full Article
