Medical University of South Carolina neurophysiologist Jessica Barley, Ph.D., and neurologist Jonathan C. Edwards, M.D., noticed a clinical problem and decided to do something about it.
The needle electrodes used to monitor a patient’s nervous system function during surgery can also pose a safety risk. Stranded uncapped needles can find their way into health care workers or even patients.
Working with the Zucker Institute for Applied Neurosciences (ZIAN), an MUSC technology accelerator, and Rhythmlink International LLC, a medical device manufacturer headquartered in Columbia, South Carolina, the team created a novel safety electrode that has the potential to reduce needle sticks.
The electrode, known as the Guardian Needle, was recently approved by the U.S. Food and Drug Administration for intraoperative monitoring (IOM). The technology has been licensed to Rhythmlink, which is ramping up production for a rollout to hospitals nationwide this autumn.
“We thought it was unacceptable and unfair that the team providing the care to the patient should be put in harm’s way by equipment that was meant to do the opposite and ensure patient safety,” said Barley, who runs the intraoperative neurophysiology program at MUSC Health and is co-inventor of the Guardian Needle. “This is how we first came up with the design.”
During high-risk surgical cases, the neurophysiology team uses IOM to monitor a patient’s nervous system. The process involves inserting approximately 40 needles throughout the patient’s body and connecting them with long wires to the IOM machine.
“IOM serves as a vital early warning system,” explained Barley. “It preserves neurologic function in real time.”
However, the setup increases the risk of needle dislocation. Currently available needles can become uncapped when dislodged from the patient’s skin. This results in a danger of needles sticking the staff while in the operating room (OR).
“We don’t have to accept that a certain number of our staff are going to get stuck by an IOM needle,” said Edwards, chief of the Integrated Centers of Clinical Excellence in Neuroscience at MUSC Health and co-inventor of the Guardian Needle. “That’s a problem, and it’s our responsibility as people in the field to solve it.”
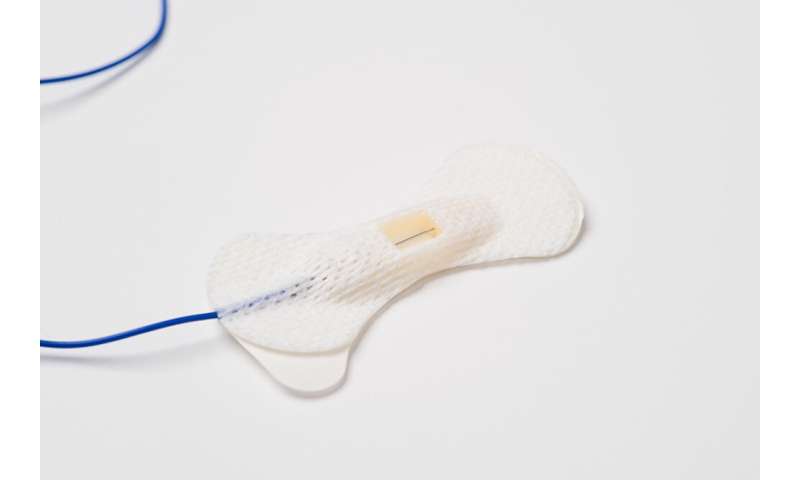
The Guardian Needle should protect the surgical team from harm because it is never uncapped. It was designed to deploy the electrode safely only when inserted in the patient. If the needle is dislodged from the skin, it automatically resheathes into its protective casing.

“The key thing is that you don’t have to cap and uncap the needle, and it automatically retracts when it’s not in the patient,” said Paul Asper, vice president of commercialization at ZIAN.
The design also includes adhesive bandages around the needles. The adhesives enable the team to secure needles to the patient without manually taping them, thus decreasing OR time and cost. The bandage, like the needle electrode, is sterile, which reduces the risk of infection from nonsterile tape.
“We did timed trials,” said Barley. “Just trying the full setup the very first time using the new design, we were all faster,” she said, comparing the new needles with the needles they had used before.
Not only does the Guardian Needle protect the surgical team and decrease OR time, but it also enables better patient care by reducing the risk of needle sticks to patients and helping to maintain a sterile environment.
The adhesives on the needle also secure it in place despite shifts in patient positioning. The adhesives thus ensure signal integrity as the electrodes monitor nervous system function during surgery.
The clinician-innovators were able to come up with the clever design because they were personally familiar with the clinical problem they were trying to address.
“Clinicians have great ideas all the time,” said Edwards. “But 99% of those ideas die, mostly because we don’t have time.”
Enter ZIAN, with the expertise, knowledge and resources to turn an idea into a product. In the case of the Guardian Needle, the ZIAN team developed a business plan and patent strategy, raised funding for research and development, engineered the prototype and forged a licensing agreement with a world-class medical device company, saving valuable time for the busy clinicians.
“The expertise on the ZIAN team aligns perfectly with the clinical expertise of the inventors, enabling both parties to execute on their strengths,” explained Mark Semler, CEO of ZIAN. The core mission of ZIAN is to develop and bring to market technologies that solve unmet clinical needs.
“We have that clinical perspective to create a pipeline of ideas,” said Edwards. “ZIAN provides the practical implementation of those ideas, and neither of those two would be successful without the other.”

Rhythmlink, a South Carolina-based company specializing in medical devices that record or elicit neurophysiologic biopotentials, has licensed the technology and has begun to ramp up production of the Guardian Needle. Their unique position in the industry allowed them to recognize the importance of this invention. That, combined with their contribution to the intellectual property, design enhancements for manufacturing and expertise in regulatory guidelines, helped the product become a reality.
“This is a great example of South Carolina organizations collaborating in the health care space and an illustration of South Carolina’s prowess in innovation, entrepreneurship, life sciences and manufacturing,” said Shawn Regan, co-founder and chief executive officer of Rhythmlink. “Creating a safer work environment for health care professionals absolutely aligns with our mission to improve patient care. Working with ZIAN and MUSC to develop the Guardian Needle and bring this creation to life was a no-brainer from a collaboration standpoint.”
Successful commercialization of the product and the widespread distribution that Rhythmlink can provide are key to realizing a potentially industry-changing standard of care. As the novel electrode is rolled out in hospitals across the country, researchers will collect needle-stick data to determine whether it is safer than the current standard of care. If it is safer, as its inventors believe, it would likely become the new standard of care, given federal workplace safety rules.
“Being at the forefront of an innovative and potentially industry-changing movement is exciting and exactly where we strive to be,” said Regan.
To the inventors, the Guardian Needle provided a way to make a difference not only for their MUSC Health colleagues but also for surgical team members across the globe.
“In health care, we gladly and eagerly place ourselves at risk every day when we’re caring for others. But it does have an element of stress and anxiety,” said Barley. “This invention is particularly special because we’re not only caring for our patients in a safer, higher-quality way, we’re also protecting our colleagues and teammates. It feels like a way of giving back to them and keeping them safe.”
Edwards explained that it is this type of innovation that has enabled him to help patients and health care providers he will never meet. This he considers a benefit of practicing academic medicine.
“We always think of clinical practice, teaching and research as the three pillars of medicine,” he explained. “There’s a fourth pillar, and that fourth pillar is innovation.”
Innovation has led this MUSC team to create a solution for a once-tolerated problem. They encourage other clinicians to do the same.
Source: Read Full Article
