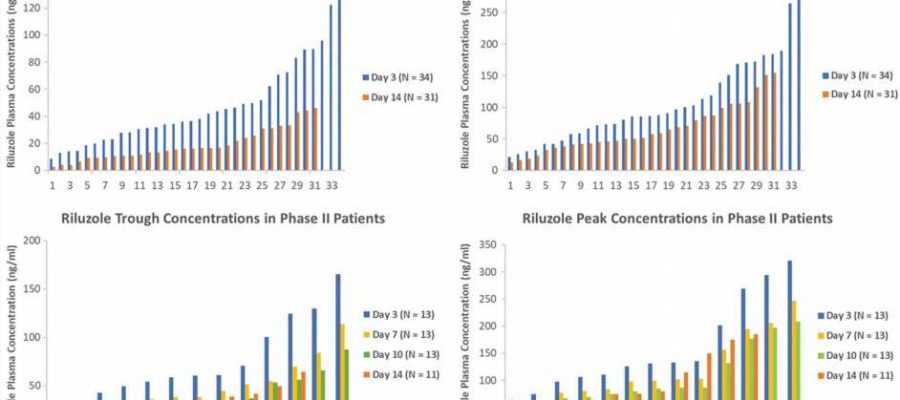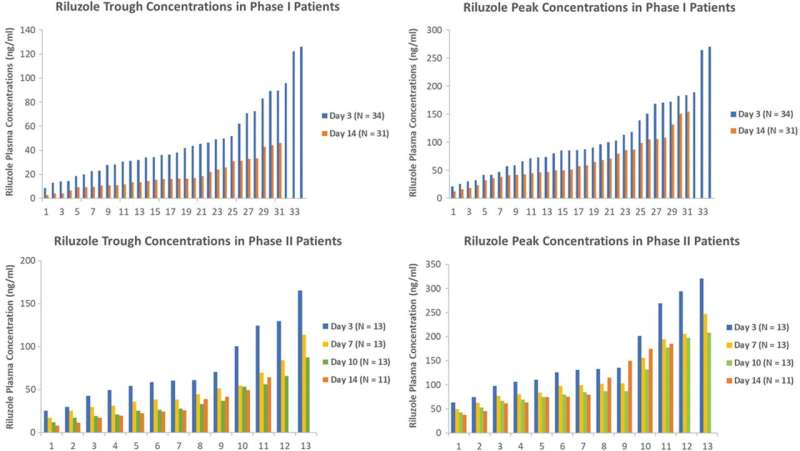
A small clinical trial with a pharmacokinetic sub-study, led by a pharmacologist at the University of Houston, has demonstrated the promising effectiveness of the drug Riluzole for improving functionality in people with acute spinal cord injuries (SCI) if the drug is taken within 12 hours post-injury.
Riluzole is among the first drugs to show efficacy for treating acute SCI, which impacts an estimated 18,000 people in the United States each year. The U.S. Food and Drug Administration (FDA) approved the drug in 1995 for the treatment of amyotrophic lateral sclerosis, known as ALS or Lou Gehrig’s Disease, with a daily oral dose of one 50-milligram tablet twice a day.
The same dosage regimen was used for this phase 2/3 multi-center clinical trial repurposing the drug for SCI patients. The work is published in the Journal of Neurotrauma.
“Riluzole is a medication that blocks certain sodium channels and is commonly used as an anticonvulsant. However, our studies demonstrate its neuroprotective potential to preserve nerve cells and help people regain some of their lost functions after spinal cord injury,” said lead study author Diana S-L Chow, Paula & John J. Lovoi Sr. Endowed Professor in Drug Discovery and Development and director of the Institute of Drug Education and Research at the UH College of Pharmacy.
Chow cautions that while the results of this study are positive, further investigation is needed given the small number of participants involved in the trial—32 patients with head and neck injuries were examined.
“The contribution of our investigation is to offer the proof of concept for the drug discovery and development approach for SCI so that the scientific community may facilitate future treatments,” said Chow, noting that Riluzole can be prescribed for “off-label” use by physicians in clinical settings for a different purpose, such as acute SCI. However, it is not for chronic SCI patients to use for purposes other than ALS, before FDA approval.
“These findings have the potential to influence future dosing strategies, ultimately enhancing patient care and improving therapeutic outcomes,” she added.
The acute and progressive nature of traumatic SCI and the complexity of secondary injury alters the pharmacokinetics of therapeutics, namely, how the body processes a drug. For the clinical trial, the researchers developed a model to capture the dynamic nature of the drug’s behavior and patient response, including motor scores in elbow flexors/extensors, wrist extensors and finger flexors/abductors in the upper limbs; hip flexors, knee extensors, ankle dorsiflexors/plantar flexors and a long toe extensor in the lower limbs.
All are influenced by the complex pathophysiology of SCI and impacts of the progression of the condition after injury.
“Our research underscores the need for a specific signal in the body that can tell us how well a treatment for spinal cord injuries works. In our study, we used an SCI-specific biomarker called phosphorylated neurofilament-heavy subunit (pNF-H) to show how Riluzole helps reduce neuron cell damage in SCI. Our findings revealed that patients who received the treatment had lower levels of pNF-H, confirming the positive effect of the medication on spinal cord injuries,” said Chow.
Chow is an internationally recognized expert in the development and analyses of new drug formulations and drug-delivery systems for the treatment of leukemia, other cancers and infection. She has also studied the stability and efficacy of medications used in space flights on the International Space Station.
This most recent study also established a link between short-term outcomes, such as pNF-H concentration, and long-term improvements in functional motor abilities. “This connection suggests the feasibility of predicting if a patient will benefit from the treatment with long-term functional improvements early in the treatment process at the bedside through the objective biomarker measurement,” she added.
More information:
Diana Shu-Lian Chow et al, Riluzole in Spinal Cord Injury Study (RISCIS)–Pharmacokinetic (PK) Sub-Study: An Analysis of Pharmacokinetics, Pharmacodynamics, and Impact on Axonal Degradation of Riluzole in Patients With Traumatic Cervical Spinal Cord Injury Enrolled in the RISCIS Phase III Randomized Controlled Trial, Journal of Neurotrauma (2023). DOI: 10.1089/neu.2022.0499
Journal information:
Journal of Neurotrauma
Source: Read Full Article