Periprosthetic joint infection (PJI) is a devastating complication of knee and hip replacement surgery that can, in some cases, be difficult to distinguish from other causes of arthroplasty failure. An “omics”-based tool that measures predicted abundance of immune cells may aid in making the diagnosis of failed arthroplasty due to PJI, suggests a study in The Journal of Bone & Joint Surgery.
A “transcriptomic-based cellular deconvolution tool” called CIBERSORTx—which allows evaluation of underlying immune responses in failed knee and hip arthroplasty cases—can distinguish PJI from non-infectious causes of complications following arthroplasty surgery, according to the new research by Robin Patel, MD, her Ph.D. student Cody R. Fisher, and colleagues at the Mayo Clinic, Rochester, Minn.
PJI samples show increases in immune cells involved in response to infection
While it is known that neutrophils are elevated at sites of infected arthroplasties, measuring neutrophil amounts has typically involved cell counting or microscopic visualization and is not possible in certain sample types. Transcriptomics is an emerging technique that uses RNA sequencing to determine which genes are expressed.
CIBERSORTx is an analysis tool for transcriptomic data that generates “predicted cellularity profiles” from RNA sequence data in samples with mixed cell populations. It provides a potential alternative to standard methods of evaluating cellular population composition from otherwise unsuitable sample types and may allow for standard methods to be foregone altogether.
Testing was performed on sonicate fluid samples, obtained using an ultrasound technique refined by Dr. Patel and her team that samples surfaces of surgically removed implants. According to Dr. Patel, “Sonicate fluid provides a direct interrogation of implant surfaces and therefore provides an ideal sample type for investigation of key immune responses during arthroplasty failure.”
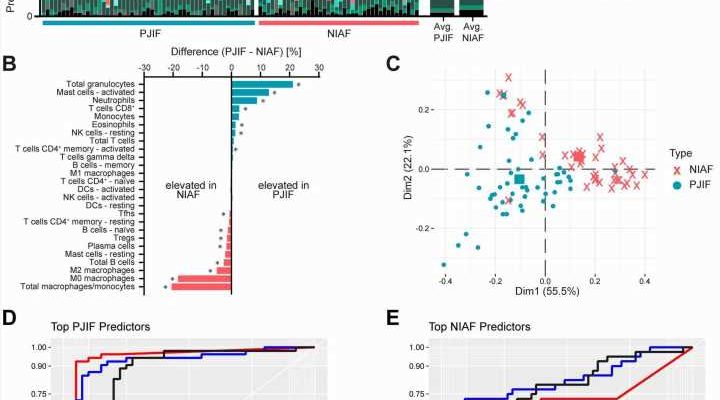
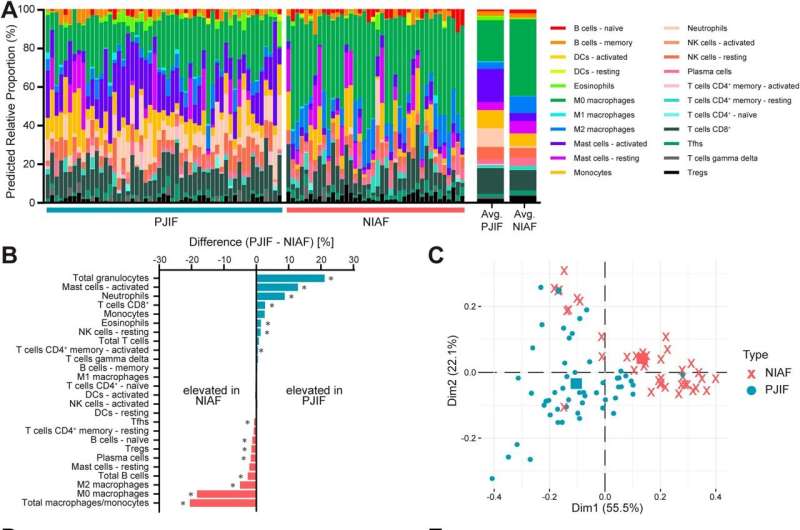
The researchers used CIBERSORTx to compare sonicate fluid samples from failed knee and hip arthroplasty components from 53 patients with PJI and 40 with non-infectious causes of arthroplasty failure. Predicted cellularity profiles were compared to determine whether the relative abundance of predicted specific cell types differed between infected and non-infected samples –thus potentially aiding in making the sometimes-difficult distinction between the two.
Of the 22 predicted cellular fractions evaluated, 12 showed differences in relative abundance in PJI samples versus those with non-infectious arthroplasty failure. Not surprisingly, samples from patients with PJI had increased proportions of immune cell types that play “crucial roles in the immune response to bacterial infection,” according to the authors. Two predicted immune cell types—neutrophils and activated mast cells—were “highly able to differentiate PJI” from non-infectious arthroplasty failure.
In contrast, non-infectious failed arthroplasty cases were associated with predicted cells involved in “tissue homeostasis and repair in response to chronic inflammation.” For example, this would include inflammation caused by osteoarthritis, rheumatoid arthritis, or immune reactions to the prosthetic device itself. In particular, predicted macrophages/monocytes, primarily those of M0- and M1-like phenotypes, were associated with non-infectious arthroplasty failure.
‘Omics’ techniques may help meet challenges of diagnosing PJI
In PJI cases, some types of immune cells were differentially abundant in Staphylococcus aureus versus S. epidermidis infections—the two most common bacterial causes of PJI.
Conventional approaches to PJI detection have limitations, as even if bacteria are detected, it may be difficult to confirm they are the cause of the patient’s symptoms. Novel “omics” approaches to investigate human characteristics (such as described in the article) and microbial characteristics (the topic of other work by Dr. Patel and her team) during infection, provide a new approach to simultaneously assess immune response to the organism causing the infection and identify it.
Dr. Patel and coauthors acknowledge some limitations of their exploratory study, including the relatively small number of patients, resulting in an inability to perform comparisons across some patient subgroups. The researchers conclude, “additional investigation in larger and more robust cohorts is needed to confirm these findings and establish potential cutoff values for future diagnostic testing.”
More information:
Cody R. Fisher et al, Sonicate Fluid Cellularity Predicted by Transcriptomic Deconvolution Differentiates Infectious from Non-Infectious Arthroplasty Failure, The Journal of Bone & Joint Surgery (2022). DOI: 10.2106/JBJS.22.00605
Source: Read Full Article