Tissue engineered vascular grafts (TEVGs) can advance surgical management of infants and children who require congenital heart surgery, by creating functional vascular conduits with growth capacity. In a new report now published in Communication Medicine, Kevin G. Blum, Jacob C. Zbinden, Abhay B. Ramachandra and a large team of multidisciplinary and multi-institutional researchers used an integrative computational-experimental approach to understand the natural history of neovessel formation in a large preclinical animal model. During the experiments they combined large animal implantation studies with in vivo imaging and histology alongside computational growth and remodeling methods. The outcomes can provide insights to TEVG (tissue-engineered vascular graft) remodeling, with important implications for clinical translation for children with congenital heart disease.
Correcting birth defects—from the lab to clinic
Congenital heart surgery is aimed at correcting birth defects and require the development of artificial blood vessels known as vascular grafts. The potential to develop a vascular conduit with capacity to grow as the patient develops holds great promise in the field of advancing congenital heart surgery. Currently, patients typically outgrow a synthetic graft and require additional surgery. By using tissue engineering, bioengineers can form a promising solution to this problem by creating a vascular conduit that grows with the patient to mitigate somatic overgrowth. Results of ongoing clinical and preclinical studies in the field have confirmed the possibility of forming living vascular conduits with TEVGs to explore biological growth potential. While multiple groups have advanced the clinical translation of TEVGs, they remain to be approved in the United States. In this work, the team of researchers, conducted a series of laboratory and computational experiments to understand how TEVGs (tissue-engineered vascular grafts) developed into fully functional blood vessels. During the experiments, they showed the presence of two phases of changes to the TEVG after implantation, including an early phase of inflammation and latter phase of mechano-mediated scaffold neotissue (new tissue) remodeling. The resulting blood vessels showed the ability to grow and respond to blood flow, much like the body’s own blood vessels. The outcomes provide insight to the process of TEVG transformation to functional blood vessels, with implications of technical transfer to clinical practice.
The computational model
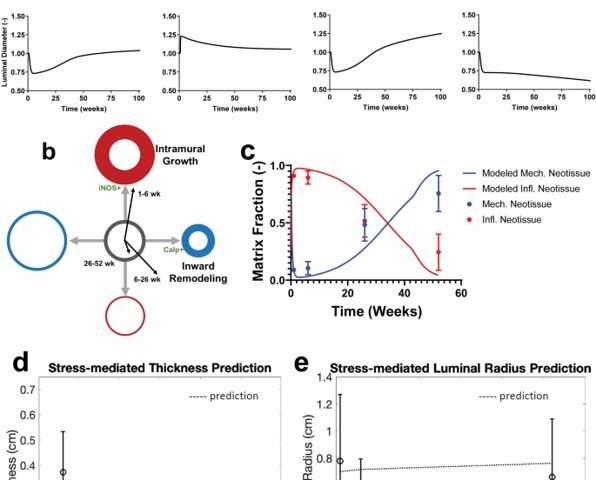
The research team built the computational model on more than a decade of experience in modeling growth and remodeling of native vessels, in response to changing mechanobiological and immunobiological stimuli. Since the TEVG model is based on an existing model originally developed to describe and predict native vessel behavior, modifications to the model relative to scaffold biodegradation, led to the hypothesis that TEVGs can transform into a neovessel that mimics native blood vessels upon scaffold degradation. During the simulations, Blum et al. tracked the mass density of each structurally significant wall constituent, including polymer, smooth muscle cells, and collagen fibers across time through several quasi-equilibrium steps and identified mass densities of these constituents into those produced via mechanobiological processes, and immunobiological processes. They simulated four case studies, and classified the behavior of TEVGs into two periods, the first referred to the period of neotissue formation influenced by the immune response of the scaffold material, followed by neovessel remodeling that occurred after scaffold degradation. A key result of the model highlighted how neovessel growth and remodeling mimicked purely mechano-mediated dynamics in native vessels.
Characterizing neovessel formation
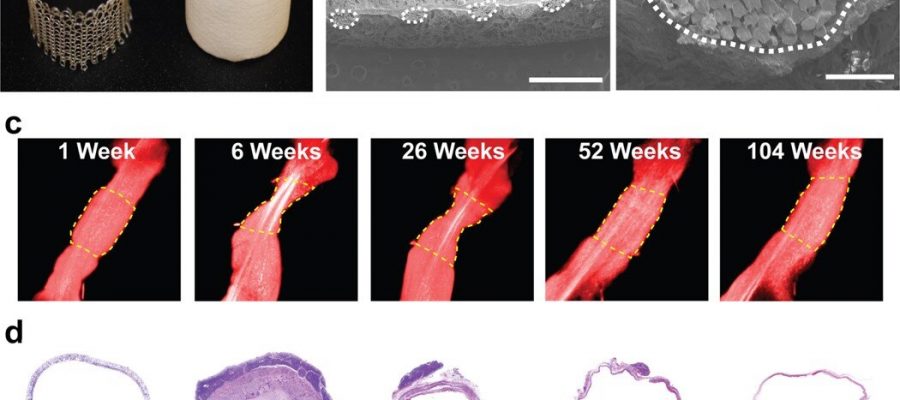
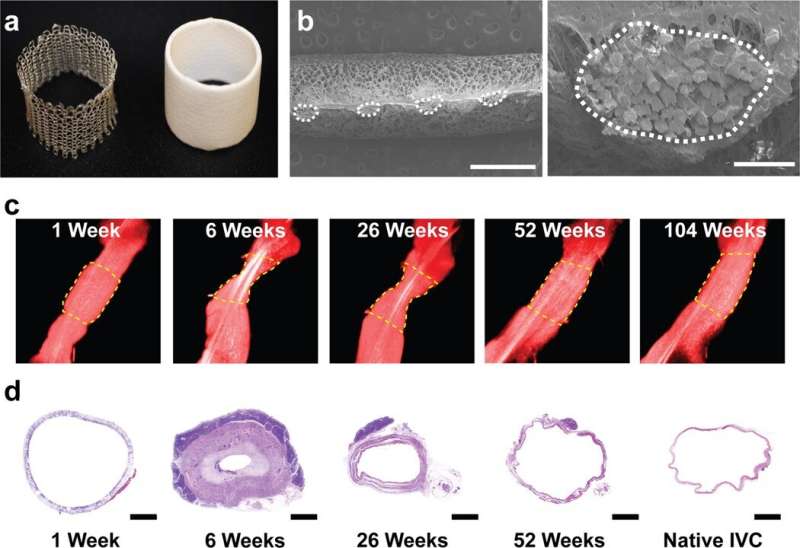
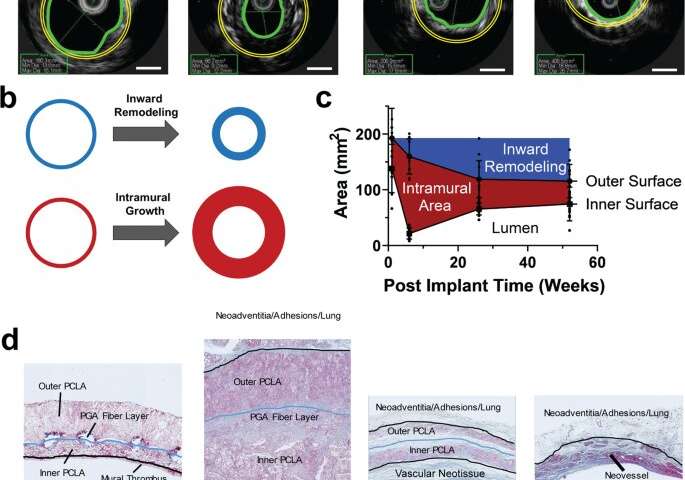
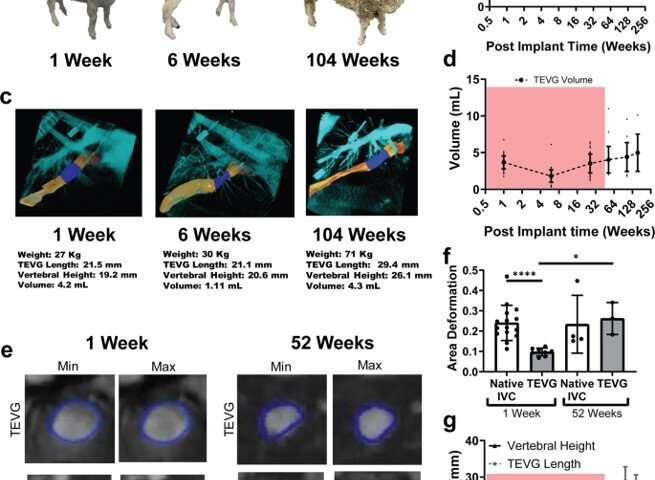
The scientists developed the scaffolds using polyglycolic acid (PGA) fibers knitted into a tube and coated with a 50:50 copolymer of polycaprolactone and poly-L-lactide to form a porous sponge. The designed scaffold could be dissolved via hydrolysis. Upon implantation in a sheep model, the team noted morphological changes in the initial 26-week period, followed by complete scaffold degradation by 52 weeks to result in a neovessel, which grossly resembled a native blood vessel. Based on serial intravascular ultrasound imaging across 52 weeks post-implantation, the team observed additional information on the morphology of evolving TEVGs, alongside histological assessments of explants at specific timeframes of interest. The team next conducted in vitro and in vivo biodegradation studies of the neovessels during growth and remodeling to examine the diminishing mass of the material, which originally provided stress shielding and inflammatory stimuli. They noted the rates of biodegradation in phosphate buffered solution up to 70 degrees Celsius and the dynamic timeframe of biodegradation in vitro and in vivo, respectively. The in-lab studies showed progressive compliance of the scaffold during polymer degradation. The scientists also examined scaffold-induced inflammation in vivo by observing the presence of markers of inflammation and credited the biomechanical properties of TEVG to material behavior and neotissue constituents.
Hemodynamics and the analysis of neotissue
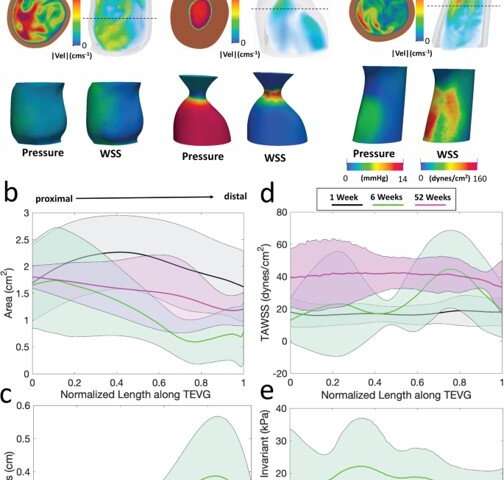
Blum et al. then assessed the effects of evolving neovessel scaffolds and their morphological changes on hemodynamics by performing subject-specific computational simulations and calculated 3D maps of major hemodynamic indices to create 3D anatomical models of TEVGs after implantation. The simulations highlighted the natural history of scaffold development in vivo from stenosis development evidenced by marked narrowing of lumen cross-sectional area, which reversed by 52 weeks. Further analyses showed the importance of extent and timing of immune- and mechano-mediated neotissue formation during scaffold evolution. Based on the simulations, Blum et al. suggested that growth and remodeling after scaffold degradation would progressively yield a neovessel that mimics native vessels. By 52 weeks after implantation, using surface scanning electron microscopy they noted continuous luminal covering of endothelial cells, and using immunofluorescent staining they envisioned the confluence of endothelial cells. The neovessels also exhibited contractile responses similar to native vessels in response to biochemical stimulation, to suggest native-like biomimetic structures with bioinspired function.
Outlook from the lab to the clinic—pediatric population
Source: Read Full Article