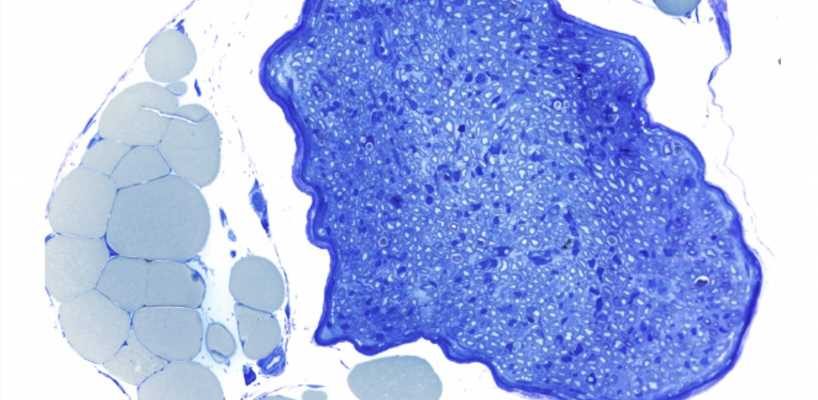
Damage to the body’s peripheral nerves can cause pain and movement disorders. Researchers at the Leipzig University have recently investigated how damaged nerves can regenerate better. They found that fat tissue strongly supports the Schwann cells needed for repair during the healing process. The results were published in the journal Cell Metabolism.
Our bodies are traversed by millions of nerve fibers that transmit information. This allows us to do things like control muscles and perceive sensory impressions. Peripheral nerves, like those in our arms and legs, are often damaged by acute injuries, for example, in accidents. As a result, those affected suffer from loss of muscle strength and sensory problems such as numbness. Peripheral nerves do have a strong regenerative potential, but complete recovery of nerve function is still rare for reasons that are not yet fully understood.
When a nerve is crushed or severed, the individual nerve fibers affected by the damage initially die. In principle, they have the ability to grow back and regenerate completely. This depends on the Schwann cells that surround the nerve fibers.
These cells do not die after nerve damage, but instead are responsible for coordinating the breakdown and regrowth of nerve fibers in their original areas. Schwann cells therefore play a key role in the repair process. It was previously unknown how these cells cope with the enormous metabolic load associated with the breakdown and rebuilding of nerve tissue.
Researchers at the University of Leipzig Medical Center have now discovered that Schwann cells receive crucial support with nerve repair from the fat tissue that surrounds nerves in the body. Using genetically modified mice, the research team has shown that the chemical messenger leptin plays a key role in this process.
Leptin is mainly produced by cells in fat tissue and is known for its appetite-suppressing effects in the context of nutrition. Surprisingly, the current research project showed that leptin signaling is also an important factor in the repair of damaged nerves by Schwann cells. “Leptin derived from fat cells stimulates the energy balance of the Schwann cells by activating their mitochondria,” explains Dr. Robert Fledrich from the Institute of Anatomy at Leipzig University who is one of the two study leaders.
“At the same time, the mitochondria of the Schwann cells use parts of the damaged nerve tissue as an energy substrate so that successful regeneration can take place,” adds Professor Ruth Stassart from the Paul Flechsig Institute of Neuropathology at the University of Leipzig Medical Center and co-leader of the study. “The metabolism of the Schwann cells is therefore optimized for nerve regeneration and significantly promotes the restoration of the original nerve function,” the two researchers explain.
The communication between fat cells and Schwann cells could potentially open up new treatment options that positively influence the metabolism of repair cells in the event of nerve damage. The researchers hope that the new findings will help to improve the regeneration of damaged nerves in humans in the future.
More information:
Venkat Krishnan Sundaram et al, Adipo-glial signaling mediates metabolic adaptation in peripheral nerve regeneration, Cell Metabolism (2023). DOI: 10.1016/j.cmet.2023.10.017
Journal information:
Cell Metabolism
Source: Read Full Article