Beta cells in the pancreas are responsible for producing the vital hormone insulin. In diabetes, these cells are either destroyed or functionally impaired, resulting in elevated blood sugar levels in the body.
Researchers led by Principal Investigator Stefan Kubicek at CeMM, the Research Center for Molecular Medicine of the Austrian Academy of Sciences, have now shown that alpha cells, which are also located in the pancreas, can be stimulated to produce insulin by targeting the chromatin protein SMNDC1. The study, published in Cell Reports, identifies a new molecular mechanism regulating the insulin hormone that plays an essential role for the treatment of diabetes
Insulin, a hormone of the pancreas, regulates blood glucose levels in the body and influences numerous metabolic processes both directly and indirectly. Beta cells are the cell type in the pancreas responsible for insulin production and secretion. In type 1 diabetes, these beta cells are destroyed by an autoimmune reaction, but also in type 2 diabetes their function is impaired. Regenerating functional beta cell mass has therefore become a main aim of diabetes research.
Beta cells are found in the pancreas, specifically within cell clusters known as islets of Langerhans. These islets also contain other hormone-producing cells—or endocrine cells—that are closely related to beta cells. One of these cell types is alpha cells, which produce glucagon, a hormone that functionally counteracts insulin. Previous research has shown that alpha cells can be converted into beta cells by genetic overexpression involving a combination of transcription factors. However, this approach cannot easily be translated into therapeutic options.
Tamara Casteels, Ph.D. student in the Kubicek lab, investigated the fundamental question of which factors repress insulin production in alpha cells, to identify factors that when inhibited could induce the expression of this important hormone. She focused on chromatin binders and modifiers, that localize directly to DNA, and thereby potentially also bind the insulin gene. Compared to transcription factors, the structures of these chromatin proteins make them theoretically easier to target therapeutically.
Loss of ATRX leads to induction of insulin
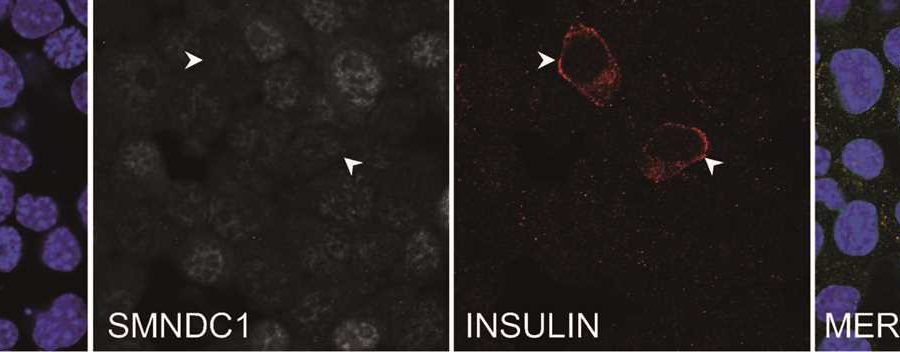
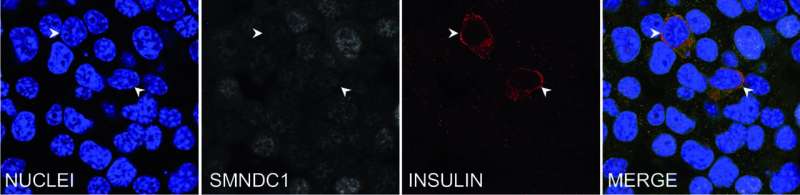
Tamara Casteels was able to show that reducing the expression of the chromatin protein SMNDC1 leads to the insulin gene being switched on in alpha cells. This effect was observed not only in a mouse cell line, but also in primary human islets of Langerhans. SMNDC1 is a known splicing factor that has not been extensively studied previously.
Stefan Kubicek explains that “splicing is a step in mRNA processing in which the non-coding intron sequences are cut out of the pre-mRNA. We have shown that the knockdown of SMNDC1 leads to splicing changes at hundreds of genes. Interestingly, one of these genes encodes the chromatin remodeler ATRX, to which SMNDC1 also binds directly. We find that loss of SMNDC1 reduces the abundance of both ATRX mRNA and ATRX protein. This, in turn, causes upregulation of the important beta cell transcription factor PDX1, which is well-known to stimulate insulin production.”
Further effects of the knock-down of SMNDC1 still open
This publication brings important fundamental insights into the regulation of insulin in alpha cells. Initial attempts to regulate SMNDC1 in beta cells further indicate that the protein also influences insulin production there.
However, before considering translational opportunities regarding potential future therapeutic use of an SMNDC1 knockdown for insulin production, many challenges remain. “The amount of insulin induced in alpha cells after SMNDC1 had been knocked-down, are significantly lower than those induced in beta cells”, Kubicek said. “And as an essential gene, complete loss of SMNDC1 can impair the viability of most cell types.”
Source: Read Full Article