In a recent study posted to the medRxiv* pre-print server, researchers evaluated the immunogenicity of booster doses of alum-adjuvanted SCB-2019 vaccine candidate's three formulations. They tested this novel vaccine candidate in Brazilian adults primed with the ChAdOx1-S coronavirus disease 2019 (COVID-19) vaccine between November 2021 and March 2022.
 Study: Homologous and heterologous boosting of the ChAdOx1-S1-S COVID-19 vaccine with the SCB-2019 vaccine candidate: a randomized, observer-blinded, controlled, phase 2 study. Image Credit: PhotobyTawat / Shutterstock
Study: Homologous and heterologous boosting of the ChAdOx1-S1-S COVID-19 vaccine with the SCB-2019 vaccine candidate: a randomized, observer-blinded, controlled, phase 2 study. Image Credit: PhotobyTawat / Shutterstock
Background
Many low- and middle-income countries (LMICs), including Brazil, are using viral vector-based vaccines (e.g., ChAdOx1-S1) for vaccinating their populations. Implementing vaccination campaigns with booster shots could help broaden the immune response amid waning immunity following vaccination and changes in the antigenic target with new waves of the COVID-19 pandemic.
SCB-2019 is a novel recombinant severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) spike (S)-protein vaccine (S-Trimer) whose two 30 μg doses demonstrated 67.2% efficacy against COVID-19 in the SPECTRA phase II/III trial. It showed an efficacy of 78.7%, 91.8%, and 58.6% against SARS-CoV-2 Delta, Gamma, and Mu variants.
About the study
In the present study, researchers investigated the use of SCB-2019 in heterologous booster regimens compared with homologous boosters. They aimed to select the optimal SCB-2019 formulation for the heterologous boosting of individuals vaccinated with the ChAdOx1-S1 vaccine. The team also assessed the safety and immunogenicity of the three formulations of SCB-2019.
The first two formulations had a 9 μg dose of SCB-2019 and alum with and without the CpG oligodeoxynucleotides (CpG-1018) adjuvant to investigate possible dose-sparing. The third formulation comprised the standard 30 μg dose with CpG-1018 and alum.
The 120 eligible participants were male or female adults over 18 years vaccinated with the ChAdOx1-S1-S vaccine six months (± four weeks) before enrolment and complied with all the study procedures and requirements, including scheduled visits and laboratory tests. At the enrolment, the researchers randomly allocated all the study participants 1:1:1:1 to receive one of the three SCB-2019 formulations.
Trained unblinded vaccine administrators prepared the final vaccine formulations. They administered the vaccine intramuscularly (i.m.) to all the study participants using one mL tuberculin syringes. The team monitored the participants who received their assigned vaccination for 30 minutes for any immediate reactions. Later, the participant self-reported occurrence of solicited local reactions, such as pain, erythema, and swelling at the injection site, and systemic adverse events, if any. They also reported any serious adverse events (SAE), or medically attended adverse events (MAAE) up to day 29 of the follow-up period.
The researchers collected serum samples before vaccination and on days 15 and 29 to assess the immune responses but tested samples from only those participants who received the correct vaccination and had no protocol deviation. They used an enzyme-linked immunosorbent assay (ELISA) to measure immunoglobulin G (IgG) antibodies against SCB-2019 S-protein. Likewise, they measured the inhibition of binding of S-protein to the human angiotensin-converting enzyme 2 (ACE2).
The team expressed ELISA antibody titers against SCB-2019 S-protein as geometric mean titers (GMT), geometric mean-fold rise in titers over baseline (GMFR), and seroconversion rates (SCR). In the study participants with a baseline titer above the lower limit of quantitation (LLOQ), a four-fold increase in post-vaccination titer indicated seroconversion. Conversely, in those who did not have detectable activity at baseline, a post-vaccination titer more than four-fold the LLOQ indicated seroconversion.
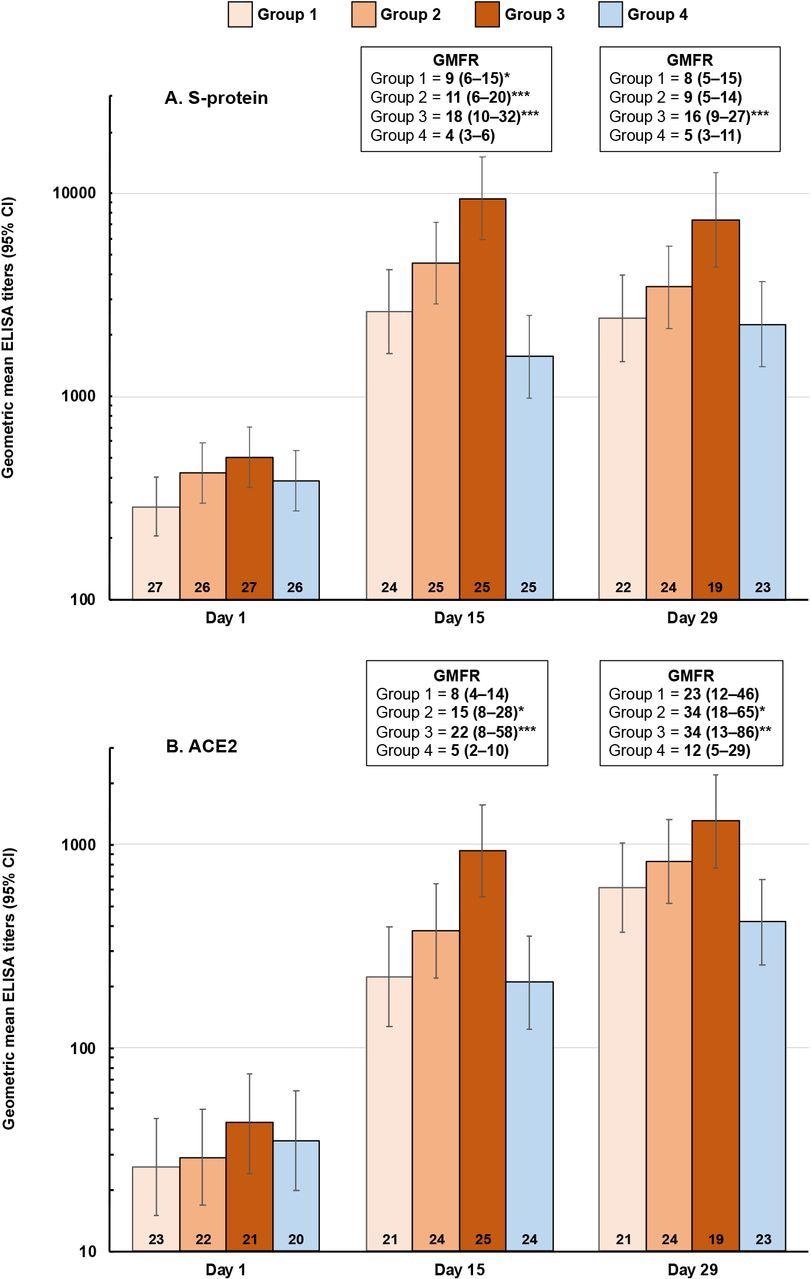
Booster vaccination responses shown as geometric mean titers (95% CI) of ELISA antibodies against SCB-2019 (panel A) and ACE2 (panel B) at Days 15 and 29 after vaccination. Geometric mean-fold rises (GMFR) from Day 1 (95% CI) are shown with ANCOVA p values of differences between Groups 1-3 (SCB-2019) and Group 4 (ChAdOx1-S): * p <0.05; ** p < 0.01, *** p < 0.001). Numbers in columns are n values per group.
Study findings
The standard formulation containing 30 μg SCB-2019 with the toll-like receptor 9 (TLR-9) agonist CpG-1018 and alum showed the best booster response. This formulation demonstrated acceptable reactogenicity, comparable to that observed in the SPECTRA study. Moreover, it was safe and highly efficacious as a heterologous booster following primary vaccination.
Additionally, the 30 μg SCB-2019 dose elicited a significantly higher immune response against SARS-CoV-2 S-protein and inhibited the binding of S-protein to the ACE2 receptor, better than the homologous ChAdOx1-S vaccine. Furthermore, it showed better neutralizing activity against four SARS-CoV-2 variants. The neutralizing activity against the SARS-CoV-2 Omicron variant was significantly lower 15 days after homologous boosting with SCB-2019+CpG+alum formulations. However, titers against Omicron waned slightly in the SCB-2019 groups by day 29.
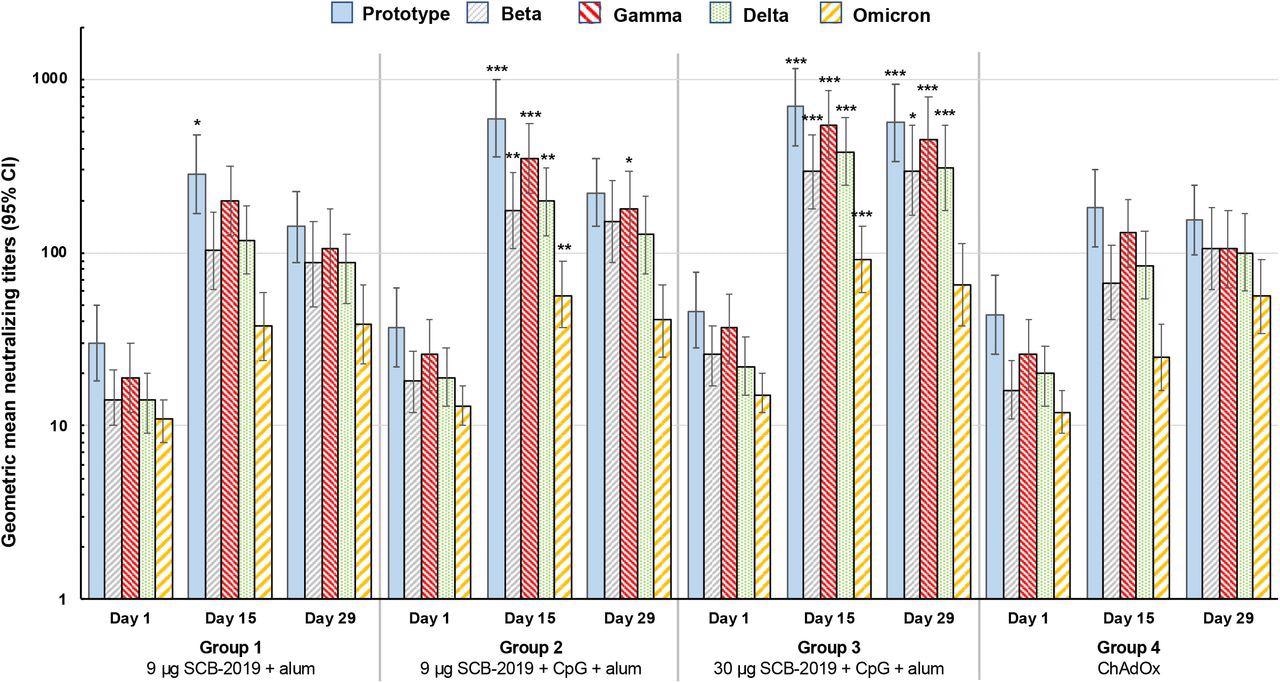
Booster vaccination responses shown as geometric mean neutralizing titers (with 95% CI) against the indicated SARS-CoV-2 variants 15 days after vaccination. Differences in GMTs of Groups 1-3 vs. Group 4 at Day 15 were tested by ANCOVA; * p < 0.05; ** p < 0.01; *** p < 0.001.
Conclusion
Overall, the 30 μg SCB-2019 formulation adjuvanted with CpG-1018 and alum was safe and well-tolerated in those previously primed with ChAdOx1-S. Importantly, it was immunologically more effective when given as a heterologous booster.
Previous studies have shown that the immune response to a heterologous second messenger ribonucleic acid vaccine after a primary dose of ChAdOx1-S was more rapid than the homologous vaccination. Therefore, further investigation is warranted to determine the difference in the kinetics of the immune response to the homologous booster of the SCB-2019 vaccine formulation.
*Important notice
medRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.v1
- Homologous and heterologous boosting of the ChAdOx1-S1-S COVID-19 vaccine with the SCB-2019 vaccine candidate: a randomized, observer-blinded, controlled, phase 2 study, Sue Ann Costa Clemens, Eveline Pipolo Milan, Eduardo Sprinz, Jose Cerbino Neto, Filippo Pacciarini, Ping Li, Hui-Ling Chen, Igor Smolenov, Andrew Pollard, Ralf Clemens, medRxiv pre-print 2022, DOI: https://doi.org/10.1101/2022.05.31.22275010, https://www.medrxiv.org/content/10.1101/2022.05.31.22275010
Posted in: Medical Research News | Disease/Infection News | Pharmaceutical News
Tags: ACE2, Angiotensin, Angiotensin-Converting Enzyme 2, Antibodies, Antibody, Assay, Coronavirus, Coronavirus Disease COVID-19, CpG, Efficacy, Enzyme, Erythema, Homologous, Immune Response, immunity, Immunoglobulin, Laboratory, Omicron, Pain, Pandemic, Protein, Receptor, Respiratory, Ribonucleic Acid, SARS, SARS-CoV-2, Severe Acute Respiratory, Severe Acute Respiratory Syndrome, Syndrome, Vaccine, Viral Vector

Written by
Neha Mathur
Neha is a digital marketing professional based in Gurugram, India. She has a Master’s degree from the University of Rajasthan with a specialization in Biotechnology in 2008. She has experience in pre-clinical research as part of her research project in The Department of Toxicology at the prestigious Central Drug Research Institute (CDRI), Lucknow, India. She also holds a certification in C++ programming.
Source: Read Full Article