Alzheimer’s disease (AD) is among the most common and distressing of dementia disorders. It is estimated that 60% to 80% of AD is heritable, which has driven a considerable amount of research to identify therapeutic gene targets that may help mitigate this condition. A new Nature Genetics study describes over 70 gene markers that appear to be significantly associated with AD risk using translational genomics.
Study: New Insights into The Genetic Etiology of Alzheimer’s Disease and Related Dementias. Image Credit: ART-ur / Shutterstock.com
About the study
In the current study, researchers used data from a new case-control study involving over 20,000 patients with a clinical diagnosis of AD, with over 22,000 controls from 15 European countries. In addition, the researchers used genome-wide association study (GWAS) data from the European Alzheimer & Dementia Biobank (EADB) consortium and the proxy-AD GWASs of the U.K. Biobank (UKBB) .
Study findings
Seventy-five different loci were identified for this disease trait, of which 33 that had been identified earlier were confirmed, while the rest were newly discovered.
The pathway enrichment analyses clarified the role of several tau-binding proteins. These included amyloid-beta peptides, which is the abnormal protein that is a major component of amyloid plaques found in people with AD, as well as amyloid precursor protein (APP) in late-onset AD.
The identification of the ADAM17 gene, which encodes an alpha-secretase, as an AD/related dementia (ADD) trait indicates possible deregulation of APP metabolism through non-amyloid pathways that ultimately lead to the accumulation of amyloid protein. The researchers also found six other genes that likely modulate APP metabolism, including ICA1, ICA1L, DGKQ, and DOC2A.
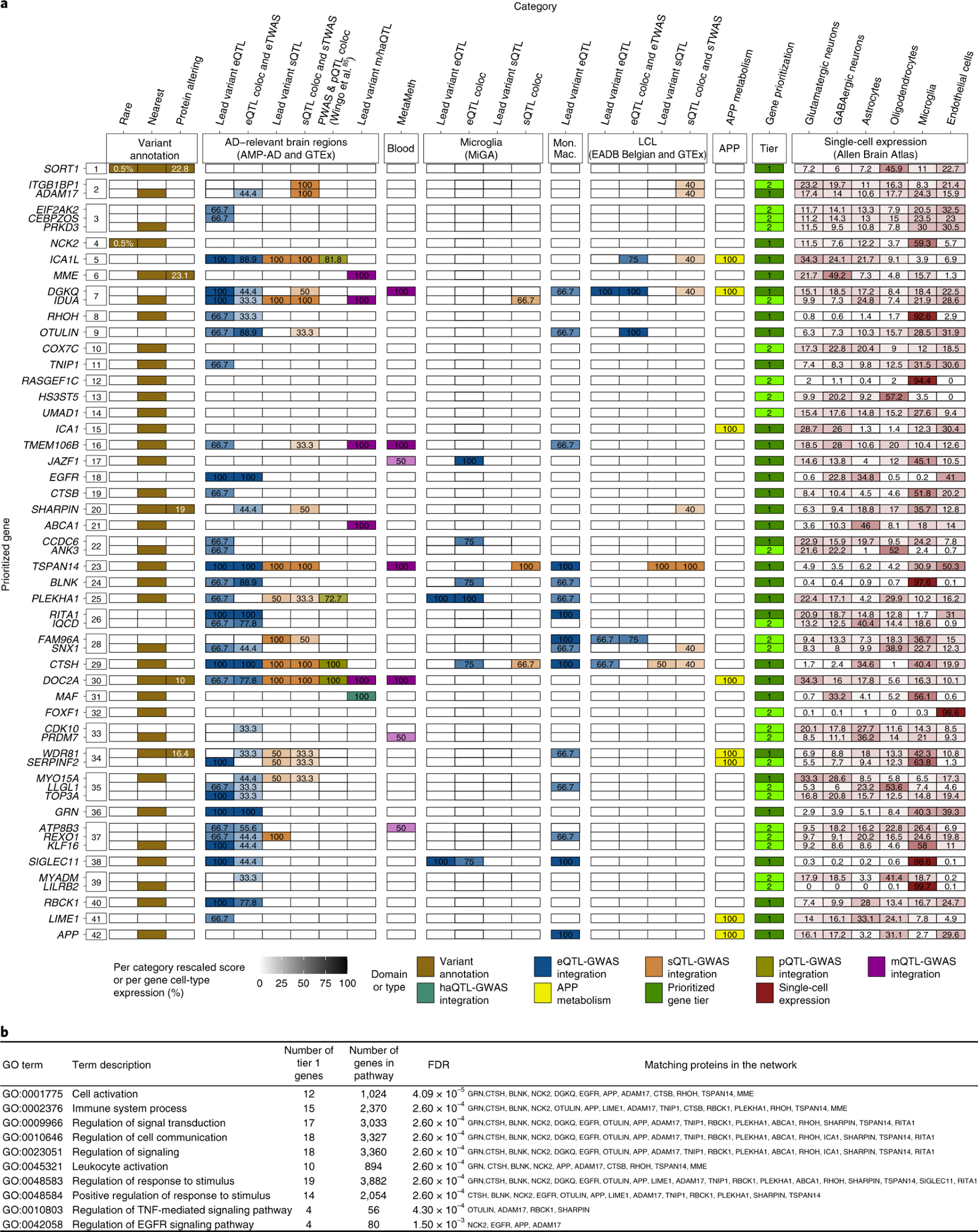 a, Summary of weighted scores for each evidence category for the prioritized genes in the 42 new genome-wide-significant loci. Using our gene prioritization method, we considered the genes within 1 Mb of each new lead variant and prioritized a total of 55 genes in 42 new loci at two different confidence levels (31 tier 1 genes and 24 tier 2 genes). The leftmost squares indicate the new locus index number. The different types of evidence are colored according to the seven different domains to which they belonged. Weighted scores for each evidence category are rescaled to a 0–100 scale, and the proportions of mean human brain cell-type-specific expression for each gene are also rescaled to a 0–100 scale; darker colors represent higher scores or higher expression proportions. Tier 1 genes are shown in dark green, and tier 2 genes are shown in light green. Only tier 1 and tier 2 genes are shown for each locus. Supplementary Fig. 35 shows full results. MAFs and CADD (v1.6) PHRED scores for rare and/or protein-altering rare variants are labeled in white within the respective squares. b, Pathway enrichment analysis based on the tier 1 gene list. Only the ten strongest associations (according to STRING software) are presented here. coloc, colocalization; eQTL, expression QTL; eTWAS, expression transcriptome-wide association study; GO, Gene Ontology; haQTL, histone acetylation QTL; Mon. Mac., monocytes and macrophages; sTWAS, splicing transcriptome-wide association study; m/haQTL, methylation/histone acetylation QTL; sQTL, splicing QTL; FDR, false discovery rate.
a, Summary of weighted scores for each evidence category for the prioritized genes in the 42 new genome-wide-significant loci. Using our gene prioritization method, we considered the genes within 1 Mb of each new lead variant and prioritized a total of 55 genes in 42 new loci at two different confidence levels (31 tier 1 genes and 24 tier 2 genes). The leftmost squares indicate the new locus index number. The different types of evidence are colored according to the seven different domains to which they belonged. Weighted scores for each evidence category are rescaled to a 0–100 scale, and the proportions of mean human brain cell-type-specific expression for each gene are also rescaled to a 0–100 scale; darker colors represent higher scores or higher expression proportions. Tier 1 genes are shown in dark green, and tier 2 genes are shown in light green. Only tier 1 and tier 2 genes are shown for each locus. Supplementary Fig. 35 shows full results. MAFs and CADD (v1.6) PHRED scores for rare and/or protein-altering rare variants are labeled in white within the respective squares. b, Pathway enrichment analysis based on the tier 1 gene list. Only the ten strongest associations (according to STRING software) are presented here. coloc, colocalization; eQTL, expression QTL; eTWAS, expression transcriptome-wide association study; GO, Gene Ontology; haQTL, histone acetylation QTL; Mon. Mac., monocytes and macrophages; sTWAS, splicing transcriptome-wide association study; m/haQTL, methylation/histone acetylation QTL; sQTL, splicing QTL; FDR, false discovery rate.
The role of innate immunity
A second important finding from this study was that innate immunity and microglial activation were confirmed to play an important role in ADD. Using single-cell expression enrichment analysis, the scientists identified genes that were expressed in microglia, including the tier 1 genes RHOH, BLNK, and SIGLEC11, as well as two-tier 2 genes LILRB2 and RASGE1FC. Over 90% of the expression of these genes was within microglia.
Both SIGLEC11 and LILRB2 have been associated with the formation of amyloid plaques and the presence of amyloid-beta peptides. Thus, the current findings associating the expression of these genes within the microglia of ADD patients is significant.
The role of LUBAC
The linear ubiquitin chain assembly complex (LUBAC) gene was also found to be involved in ADD. Two of the three complementary LUBAC expressions are encoded by SHARPIN and RBCK1, with OTULIN playing a regulatory role. Interestingly, all three of these genes were identified as prioritized genes in this study.
LUBAC is the only E3 ligase that forms linear chains of ubiquitin using the N-terminal methionine residue of ubiquitin. Ubiquitination is a key post-translational modification process that regulates protein quality and quantity, thus making it vital for cell health. This function is known to be active in inflammation, thus contributing to innate immune responses and immunity against intracellular pathogens.
In fact, the NLRP3 inflammasome, which is a crucial part of the innate immune system, can be activated only in the presence of LUBAC, which makes it a vital component of innate immune regulation. NLRP3 inflammasome activity is key to amyloid-beta development in mouse models. By activating microglia, NLRP3 may also be responsible for tau protein disease.
LUBAC also participates in autophagy, which is the cellular self-digesting debris clearance system that recycles material and keeps the cell clean. Autophagic clearance could be induced by the modification of certain neuronal cytoplasmic inclusions by linear ubiquitin chains. This process also regulates tumor-necrosis factor α (TNF-α) pathways, though the ADAM17, which is otherwise known as the TNF-α-converting enzyme, is also involved in the activation of this cytokine signal.
TNF-α regulation
The TNF-α pathway, which is among the best-known immune-inflammatory signaling pathways, is also involved in other genes that have been identified as part of the ADD gene markers. Some of these include inhibitory genes TNIP1 and PGRN, as well as SPPL2A, which encodes a protein involved in an alternative TNF-α shedding pathway. Such inhibition could reduce both amyloid-beta and tau protein disease, as suggested by earlier research.
This pathway is important for various brain functions, including the plasticity of neuronal synapses and the pathological loss of brain synapses. Furthermore, the TNF-α pathway also interacts with the EGFR pathway, which may indicate that these two pathways are interlinked during the process of ADD.
A continuum of neurodegenerative disease?
Six of the tier 1 genes in this study were not expressed at a high level in the microglia, which shows that many other cell types are involved in ADD pathology. In fact, some of the same gene variants are causally associated with frontotemporal dementia.
Ignoring the possibility that this is because some cases are wrongly classified, it is clear that genes like GRN and TMEM106B are known to be associated with multiple neurodegenerative diseases. In fact, they may represent genetic risk factors for cerebral cortical aging and cognitive impairment in conditions like Parkinson’s disease.
These genes are known to be linked to the diseased neuronal states in AD. Therefore, it is quite possible that there is a spectrum of neurodegenerative diseases with the underlying common mechanisms that are mediated by genes like GRN and TMEM106B, perhaps through their role in impaired endosomal and lysosomal trafficking and functionalities, which is a role that has been reported in AD as well.
Building a risk score
The researchers used a genetic risk score (GRS) that was constructed from the sum of the GWAS significant variants identified here. This score was then applied to population-based cohorts to detect an association with the risk of new AD and the risk of progression from mild cognitive impairment (MCI) to AD over time. Interestingly, the GRS affected the AD risk independently from the age-derived risk, with an additive impact, and was also independent of the apolipoprotein E (APOE)-derived risk.
Including the GRS into the predictive model enhanced the prediction accuracy for progressive disease, despite the fact that the effect size for the GRS-AD association was small. The increased risk between the lowest to the highest decile was almost double when the GRS was included.
The new risk variants described in this study are associated with progression to AD. Taken together, these findings help differentiate characteristics between those who are at risk of progression to dementia as compared to others.
Implications
Other scientists have postulated that there are less than 100 causal variants common enough to be significant in explaining the entire AD risk. If so, the current study has contributed a major share of these variants. However, unknown loci may still modulate the risk of AD through commonly occurring variants.
Although some rare variants may be missed due to under-representation in the available samples, almost all known rare variants are associated with common variants. There is much work that must still be conducted to elicit the network of gene-gene and gene-environment interactions that determine AD risk.
Larger GWAS studies with more people, including many more with different ancestries, will help in the development of GRSs that work better for specific groups. Such work could eventually help develop gene therapies and personalized medicine in the prevention and treatment of AD.
- Bellenguez, C., Kucukali, F., Jansen, I. E., et al. (2022). New Insights into The Genetic Etiology of Alzheimer’s Disease and Related Dementias. Nature Genetics. doi:10.1038/s41588-022-01024-z.
Posted in: Genomics | Medical Research News | Medical Condition News | Disease/Infection News
Tags: Aging, Alzheimer's Disease, Apolipoprotein, Autophagy, Brain, Cell, Cytokine, Dementia, Enzyme, Frontotemporal Dementia, Gene, Genes, Genetic, Genetics, Genome, Genomics, Immune System, immunity, Inflammasome, Inflammation, Intracellular, Ligase, Medicine, Metabolism, Methionine, Microglia, Necrosis, Neurodegenerative Disease, Neurodegenerative Diseases, Pathology, Peptides, Personalized Medicine, Protein, Research, Tau Protein, Tumor, Ubiquitin

Written by
Dr. Liji Thomas
Dr. Liji Thomas is an OB-GYN, who graduated from the Government Medical College, University of Calicut, Kerala, in 2001. Liji practiced as a full-time consultant in obstetrics/gynecology in a private hospital for a few years following her graduation. She has counseled hundreds of patients facing issues from pregnancy-related problems and infertility, and has been in charge of over 2,000 deliveries, striving always to achieve a normal delivery rather than operative.
Source: Read Full Article