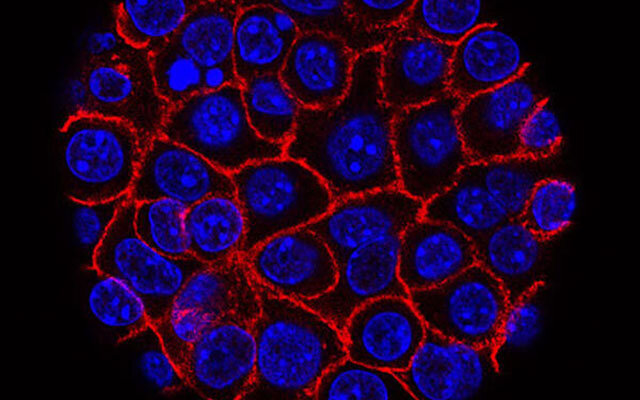
Virginia Tech researchers from the Department of Biomedical Engineering and Mechanics and the Department of Biochemistry have discovered a characteristic of a common oral bacterium that relocates to pancreatic cancer tumors that may help guide future therapeutic interventions for treatment. The bacterium, Fusobacterium nucleatum, may play a key role in how aggressively cancer grows and moves throughout the body.
Pancreatic cancer is the third-leading cause of cancer-related death in the United States. One particularly aggressive form of pancreatic cancer, pancreatic ductal adenocarcinoma, has a survival expectancy of fewer than six months.
Several characteristics make the disease difficult to treat, including its ability to suppress the immune system and its complex location and structure, which complicate surgery and chemotherapy delivery.
Scott Verbridge, associate professor in biomedical engineering and mechanics, and Barath Udayasuryan Ph.D., an alumnus from the Virginia Tech-Wake Forest University School of Biomedical Engineering and Sciences, have conducted research on a bacterium found in pancreatic cancer tumors, among other types. Most notably, they discovered ways in which the bacterium may directly impact cancer progression and resistance to chemotherapy treatments.
These results are featured in the Oct. 18 issue of Science Signaling.
Daniel J. Slade, associate professor in biochemistry and leading expert in microbes in cancer and their biochemical interactions with the tumor’s microenvironment, also collaborated with Verbridge and Udayasuryan.
Verbridge’s Laboratory of Integrative Tumor Ecology has been collaborating with Slade’s team for years on cancer research. Together, they have made discoveries on the role of a specific microbe, F. nucleatum, in driving cancer cell migration, particularly in colorectal cancer.
Because this microbe is a common oral bacterium, it has often been studied in relation to mouth diseases such as periodontitis and gingivitis. But little was known about how the microbe travels to and adapts to living within tumor microenvironments, thereby increasing the aggressiveness of cancerous growths. Other cancer research had verified the microbe’s presence in pancreatic cancer, leading Verbridge and his team to wonder if this bacterium also might be activating tumor migration in the pancreas.
“The tumor microbiome can affect the progression of cancer, so our goal is to better understand the role of these bacteria in cancer,” said Udayasuryan.
“Only in early 2022 was the tumor microbiome patently recognized as a hallmark of cancer. Cancer biology and infection biology were usually considered disparate fields of study, but recent merging of the two fields is revealing fundamental insights into cancer progression. Our focus is at the forefront of this emerging paradigm, and we are on the cutting edge of research, looking at things no one has before.”
When first analyzing the migration of infected pancreatic cancer cells, the researchers ran into an unexpected hurdle: They found that the number of migrated cells was difficult to quantify, as the total seemed to drastically outnumber the population of cells they expected to find in the system.
Using in vitro tumor-on-a-chip models, Verbridge and his team confirmed that this microbe can bind and invade the pancreatic cancer cells, which then secrete molecules that stimulate accelerated growth of cancer cells. This finding explained why the team was seeing so many more cells in their experiments than they expected. It also enabled them to identify an increase in the migration of infected cells.
In another critical discovery, they found that the microbe can infect non-tumorous, normal pancreatic tissue cells. When a normal cell was infected in their experiments, it continued to grow as normal; however, its presence stimulated nearby cancer cells to grow and spread more rapidly.
This new insight expands current ideas about non-cancerous cells in and around the tumorous cells and how cancer spreads so aggressively. Any cell infected by the microbe could potentially be more susceptible to cancerous growth at some later point, or even more prone to metastasis, which is how most cancers end up killing their hosts.
Equipped with the understanding of how bacterium in tumors are affecting the growth and spread of cancer, scientists could design more effective chemotherapy or immunotherapy treatments. These results can also aid in development of diagnostic and prognostic tools to help detect cancer earlier.
“While we have shown that F. nucleatum is capable of driving pancreatic cancer cell proliferation and migration, we do not yet know to what extent these outcomes translate to living systems or human patients,” said Verbridge. “These next steps will be important future work, which could ultimately teach us whether or not this knowledge could lead to more effective therapies that are tailored to a patient’s own microbiome components.”
“Host-microbiome interactions are complex, because many bacterial residents actually support human health and have been shown to enhance the efficacy of cancer treatments,” said Verbridge.
Source: Read Full Article