A study conducted by researchers in Qatar has found that Pfizer-BioNTech’s coronavirus disease 2019 (COVID-19) vaccine was more protective against infection with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) among individuals who had previously been infected with the virus than among those who had not.
Pfizer-BioNTech’s BNT162b2 vaccine reduced the SARS-CoV-2 incidence rate among previously infected individuals by 85% (6.6-fold), compared with among BNT162b2 recipients who had not experienced prior infection.
However, no such effect was observed for individuals who received Moderna’s mRNA-1273-vaccine, says Laith Abu-Raddad from Cornell University in Doha and colleagues.
“Prior infection enhanced protection of those BNT162b2-vaccinated, but not those mRNA1273-vaccinated,” writes the team. “These findings may have implications for the potential need of a booster vaccination.”
A pre-print version of the research paper is available on the medRxiv* server, while the article undergoes peer review.
.jpg)
The effect of prior infection on vaccination is poorly understood
The effect that previous SARS-CoV-2 infection has on the efficacy of COVID-19 vaccines in protecting against future infection remains poorly understood.
In Qatar, the COVID-19 immunization program began on December 21st, 2020. Pfizer-BioNTech’s BNT162b24 product was the first to be rolled out, followed by Moderna’s mRNA-1273 vaccine.
However, Qatar still experienced two epidemic waves between January and June 2021, dominated by the SARS-CoV-2 alpha (B.1.1.7) and beta (B.1.351) variants of concern.
“This provided an opportunity to assess whether persons vaccinated after a prior SARS-CoV-2 infection have better protection against future infection than those vaccinated without prior infection,” says Abu-Raddad and colleagues.
Testing the effects of prior infection in two national studies
The team assessed two national retrospective matched-cohort studies – one cohort that had received two doses of BNT162b2 and one that had received two doses of mRNA-1273.
Between December 21st, 2020, and June 6th, 2021, the incidence of documented SARS-CoV-2 infection (as confirmed by polymerase chain reaction [PCR] testing) in each cohort was compared between those with and without infection prior to vaccination.
Cohorts were matched by sex, five-year age group, nationality, and calendar week of the first vaccine dose to control for differences in exposure risk and in exposure to variants.
The estimated incidence rate among those who receivedBNT162b2 was 1.66 per 10,000 person-weeks among those with prior infection, compared with 11.02 per 10,000 person-weeks among those without prior infection.
The corresponding estimated rates for those who received mRNA-1273 were 1.55 and 1.83 per 10,000 person-weeks.
For the BNT162b2 cohort, the cumulative infection incidence among those with and without prior infection was estimated to be 0.14% versus 0.93%, compared with 0.06% versus 0.08% for the mRNA-1273 cohort.
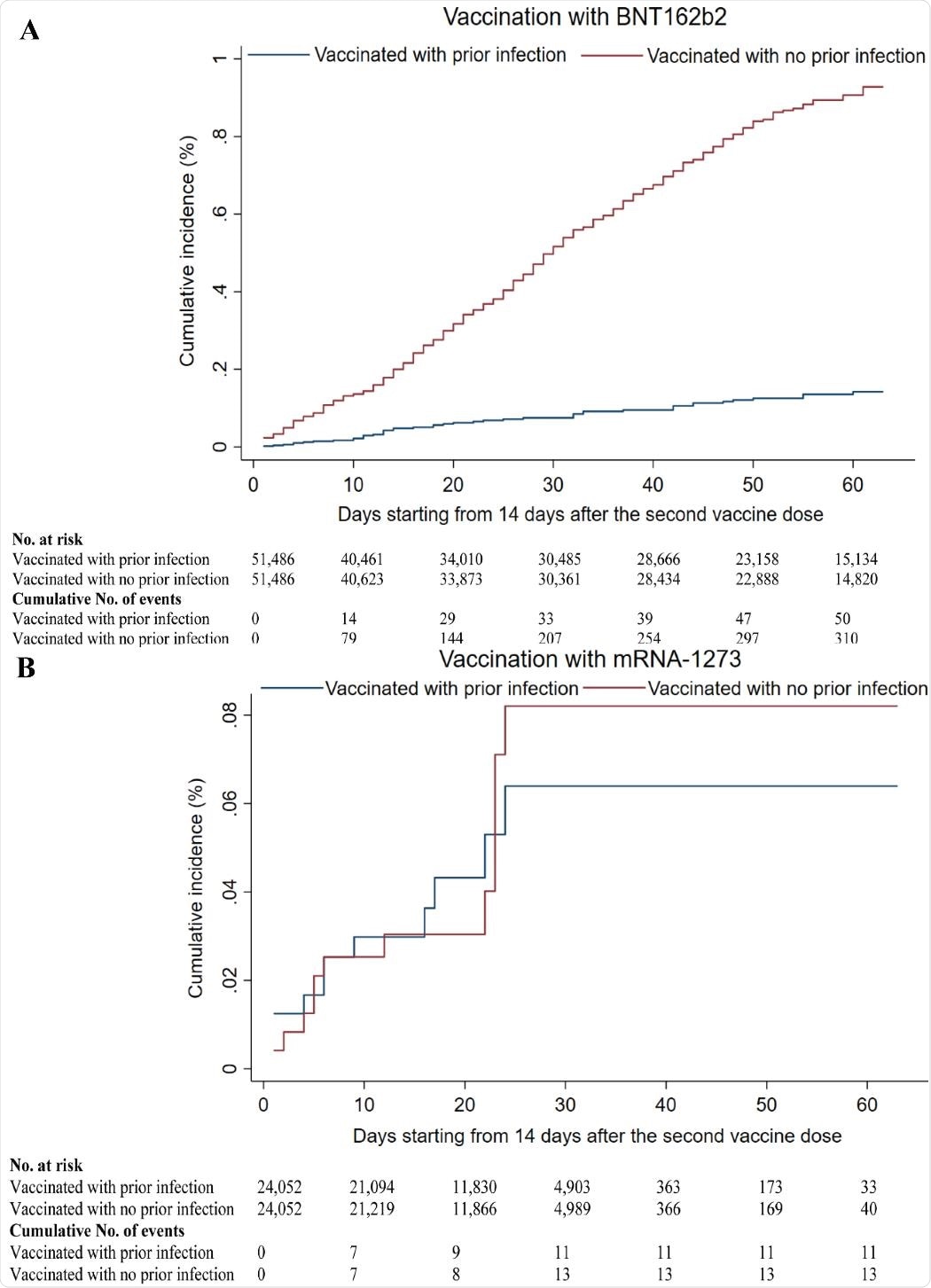
Prior infection reduced incidence by 85% in the BNT162b2-vaccinated cohort
The team says that infection incidence was low in these cohorts during a time of intense incidence in Qatar, suggesting that both vaccines were highly effective against the B.1.1.7 variant and the B.1.351 variants of concern that were dominating at the time.
“Still, prior infection of those BNT162b2-vaccinated further enhanced protection and reduced the incidence rate by 85% (6.6-fold) compared to those without prior infection,” say the researchers.
“In contrast, those vaccinated with mRNA-1273 were as well protected as those who received the vaccine after a prior infection.”
Explanations for the differences in efficacy
Abu-Raddad and colleagues suggest that the findings may be explained by the observed differences in the effectiveness of these two vaccines against the B.1.1.7 and B.1.351 variants.
In Qatar, the respective vaccine efficacies against these variants were reported to be 89.5% and 75.0% for BNT162b2, and 100% and 96.4% for mRNA-1273.
These differences in efficacy could have arisen for various reasons including differences in dosing, in the dosing interval and the mechanisms of action.
For example, BNT162b24 was administered at 30-μg per dose, while mRNA-12735 was administered at 100μg per dose.
“This may have resulted in a more activated immune response for the mRNA-1273 vaccine than the BNT162b2 vaccine, and made the existence of prior immunity due to natural infection of no additional benefit for the mRNA-1273 vaccine,” suggests the team.
Furthermore, the interval between doses was one week longer for mRNA-12735 than for BNT162b2 and studies have previously suggested that a longer dose interval could be associated with improved protection after administration of the second dose.
What are the study implications?
“Our results demonstrate low infection incidence among those vaccinated with BNT162b2 or mRNA-1273, but among those vaccinated with BNT162b2, protection against infection was further enhanced and infection incidence was further reduced by prior infection,” say the researchers.
These findings may have implications for dosing, the interval between doses, and the potential need for booster vaccination, they conclude.
*Important Notice
medRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
- Abu-Raddad L, et al. Protection afforded by the BNT162b2 and mRNA-1273 COVID-19 vaccines in fully vaccinated cohorts with and without prior infection. medRxiv, 2021. doi: https://doi.org/10.1101/2021.07.25.21261093, https://www.medrxiv.org/content/10.1101/2021.07.25.21261093v1
Posted in: Medical Research News | Disease/Infection News
Tags: Coronavirus, Coronavirus Disease COVID-19, Efficacy, Immune Response, immunity, Immunization, Polymerase, Polymerase Chain Reaction, Research, Respiratory, SARS, SARS-CoV-2, Severe Acute Respiratory, Severe Acute Respiratory Syndrome, Syndrome, Vaccine, Virus

Written by
Sally Robertson
Sally first developed an interest in medical communications when she took on the role of Journal Development Editor for BioMed Central (BMC), after having graduated with a degree in biomedical science from Greenwich University.
Source: Read Full Article
