A research team led by the University of Adelaide, in partnership with medical technology company Fertilis, has delivered a ground-breaking new micro-device to streamline the only fertility treatment procedure available for men with low sperm counts.
The first-of-its-kind device will allow more IVF clinics to offer Intracytoplasmic Sperm Injection (ICSI) as a treatment, while several IVF procedures, such as embryo culture, embryo cryopreservation and in vitro maturation, will also be improved by using the device.
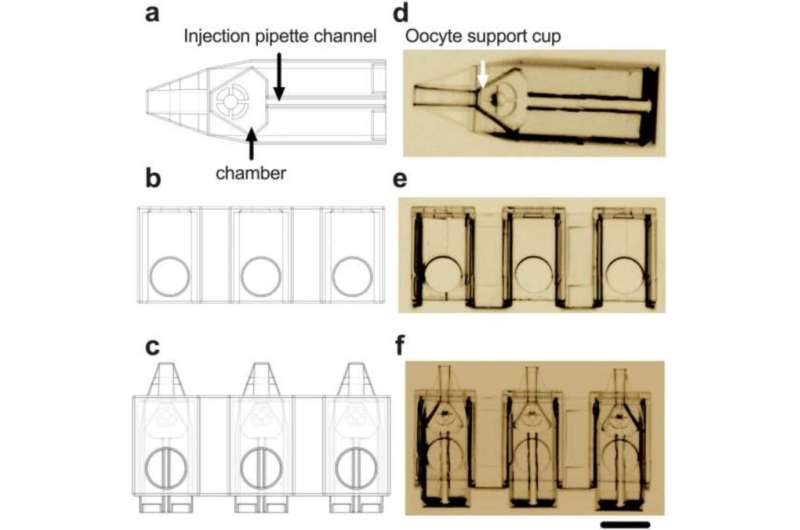
ICSI is a slow and difficult procedure that involves the injection of a single sperm into an egg for fertilization, and it can only be carried out by experienced embryologists.
This new technology—smaller than a pinhead in size—holds up to 10 eggs in segregated positions for quicker injection, making it easier for embryologists to track and avoid the risk of errors.
Lead researcher Dr. Kylie Dunning, from the University of Adelaide’s Robinson Research Institute, said the device will cut treatment time in half, require less training for embryologists with less expensive equipment than current ICSI treatment and improve access to the procedure for more patients.
“The development of this new, innovative approach is an important breakthrough for people wanting to start a family who haven’t been able to due to male infertility,” Dr. Dunning said.
“By removing the need for the pipette that normally holds the unfertilized egg in position during ICSI, this device simplifies the injection process, reduces dependency on a high level of technical experience and will dramatically improve embryo production.
“This discovery removes significant barriers to treatment for people with infertility and will improve IVF success.”
Device inventor and Fertilis co-founder, Professor Jeremy Thompson, said his company is excited to bring the breakthrough device to market.
“Where IVF science has excelled, technology has tended to stagnate—until now,” Professor Thompson said.
“ICSI hasn’t changed since its discovery 30 years ago. Continued innovation in the IVF lab like this is the only way we will boost success and reduce the financial and emotional burden for patients.”
The device will undergo global clinical trials in 2022.
This cutting-edge development would not have been possible without the support of the Australian Research Council and The Hospital Research Foundation Group.
Paul Flynn, Chief Executive Officer of the Hospital Research Foundation Group, said the organization has been proud to support Dr. Dunning’s research during the past three years to improve IVF success rates.
“This device is set to be a game-changer for thousands of hopeful parents who need to rely on ICSI,” Flynn said.
Primary author Suliman Yagoub is a Ph.D. candidate in the School of Biomedicine at The University of Adelaide.
Source: Read Full Article
