Introduction
How are nanofluids used in drug delivery?
What unique applications do nanofluids offer?
How are nanofluids directed to the target site?
References
A nanofluid is a liquid containing nanoparticles, usually colloid or a colloidal suspension. Nanoparticles may be composed of a wide variety of organic and inorganic materials, and those constructed of rolled carbon sheets or gold have popularly seen use in biomedicine. The fluid in which nanoparticles are suspended is typically water, though various oils may instead be used depending on the application.
The term nanofluid is usually encountered in industry-focused fields where the unique and emergent properties of whole nanofluids with regard to heat transfer, lubrication, and applications in petroleum refining can be exploited. For these applications, the properties of the nanofluid are defined by the nanoparticle content and liquid medium, though in biomedicine, the nanofluid acts simply as a medium in which the nanoparticles can be suspended stably and safely applied to living organisms. Some of the key features of nanofluids and their applications in biomedicine will be discussed below.

Image Credit: Billion Photos/Shutterstock.com
How are nanofluids used in drug delivery?
Nanoparticles may be utilized in a wide variety of medical applications and are being explored as drug delivery and diagnostic agents. One of the most promising uses of nanoparticle drug delivery agents is in the improved bioavailability, biocompatibility, and biodistribution profile of many drugs following incorporation onto a nanocarrier, especially where drug solubility is otherwise a limiting factor in bioavailability.
Lipid nanoparticles have been widely exploited to enhance the delivery of poorly water-soluble drugs, where the drug can be dissolved into the body of the nanoparticle to generate a colloidal suspension in water. Once in the body, lipid nanoparticles can merge with and cross cell membranes or otherwise enter cells by other mechanisms such as endocytosis, delivering the drug directly into the cell.
Other biomolecular cargo that would otherwise not survive traversal through the bloodstream has been loaded onto nanoparticle carriers, such as therapeutic and diagnostic RNA or DNA strands or functional enzymes and proteins. Recently, several vaccines have utilized lipid nanoparticles to deliver highly sensitive mRNA strands into cells with great success, wherein the cell's own machinery was co-opted to produce the spike protein of the SARS-CoV-2 virus and induce an immune response.
Following their synthesis, many nanomaterials are unstable and would quickly aggregate without additional protection, which is often provided by the presence of surfactant molecules or capping agents in the nanofluid that provide physical and electrostatic separation to the particles. However, many of the most widely employed capping agents, such as sodium dodecyl sulfate (SDS) and ethylenediaminetetraacetic acid (EDTA), are toxic in vivo, and besides which, if not bound to the nanoparticle surface would quickly be separated from the nanofluid medium following administration. Instead, nanomaterials that require further stabilization are coated with more permanently bound capping agents with high biocompatibility and water solubility, typically polyethylene glycol, which has been described as granting stealth properties to the nanomaterial by preventing interaction with surrounding proteins and assisting in avoiding early excretion.
What unique applications do nanofluids offer?
 How can we use AI to Preserve Privacy in Biomedicine?
How can we use AI to Preserve Privacy in Biomedicine?The varied and unique properties of nanomaterials may be exploited in numerous ways depending on the intended biomedical application, and beyond use as small molecule drug and therapeutic agent delivery vehicles can provide further utility as theranostics themselves. Nanoparticles constructed of metallic gold participate in an optical phenomenon known as localized surface plasmon resonance, wherein in-phase oscillations of the electron cloud surrounding the particle with the light of a specific wavelength cause extremely amplified light adsorption at that wavelength.
Antibodies eBook

The size, shape, and specific material content of gold nanoparticles determines the precise surface plasmon resonance wavelength, which is highly tunable into the near infra-red region, the wavelength of light most penetrating through biological tissue. Given this, gold nanoparticles have applications as diagnostic agents that can indicate the location of, for example, a tumor to which the nanoparticles have been localized.
Further, higher energy laser light of the surface plasmon resonance wavelength can excite and heat the particles rapidly, releasing the drug cargo and inducing localized hyperthermia. Similarly, magnetic nanoparticles constructed of iron oxide have also been produced that can be localized to the target site by magnetism and subsequently triggered to release their drug cargo and induce quickly localized hyperthermia by magnetic heating.
The high density of both metallic gold and iron oxide nanoparticles, amongst others with high z numbers, makes them excellent contrast agents for use in imaging applications such as X-ray imaging or computed tomography, while magnetic nanoparticles additionally have applications as magnetic resonance imaging contrast agents.
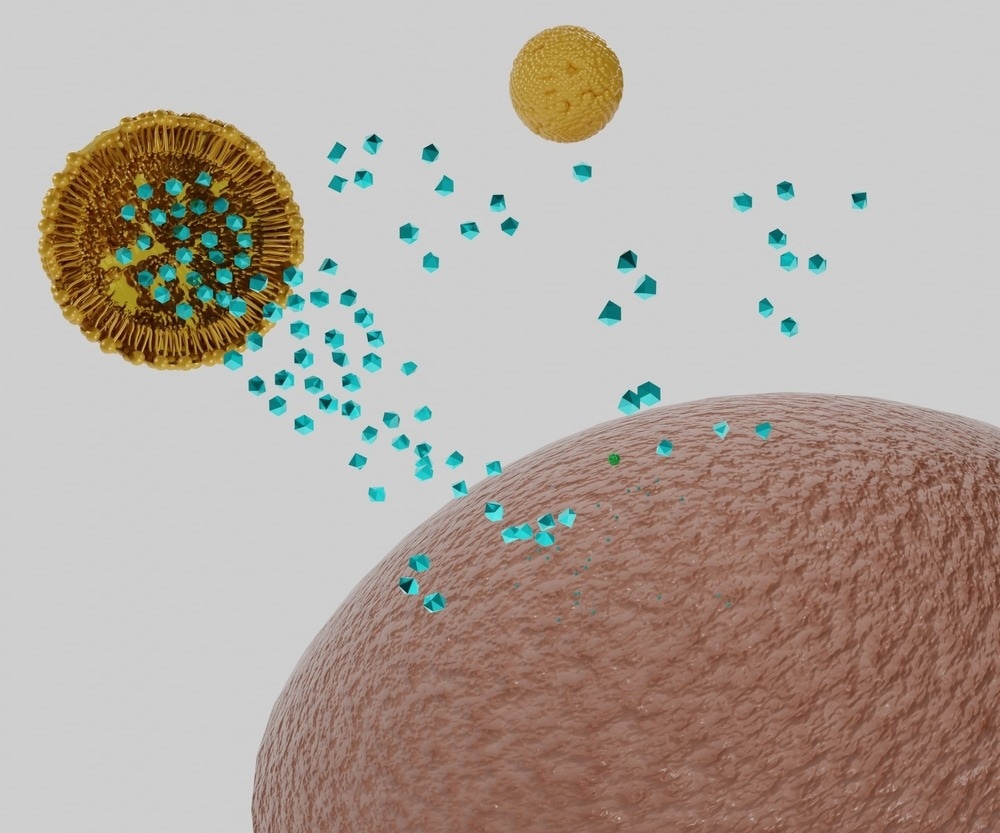
Image Credit: Love Employee/Shutterstock.com
How are nanofluids directed to the target site?
As discussed, the use of nanofluids as delivery agents and theranostics relies on their ability to localize to the target delivery site. In some cases, the target site may be non-specific, such as the aforementioned mRNA vaccines that can be effectively delivered to any cell, or may be highly specific, such as when attempting to deliver chemotherapeutic drugs to the site of a tumor exclusively.
The blood vessels surrounding heavily inflamed tissue and tumors become highly disordered, bearing cavities and fenestrations with poor blood flow. In some cases, nano-sized objects have demonstrated a propensity to accumulate in these regions in a phenomenon known as the enhanced permeability and retention effect, which provides a passive targeting mechanism to nanofluids seeking out these regions.
Incorporating active targeting functionality can further enhance the specificity of the nanoparticle, which exploits receptors at the target site to enable cell entry. For example, tumors frequently overexpress a particular receptor on the cell surface as a result of their disordered genome, and if this receptor can be identified, then the complimentary molecule can be bound to the nanoparticle.
When the nanoparticle encounters the target cell, the receptor will bond with it and drag it into the cell, where theranostics functionality can be enabled.
Primarily, targeting strategies and nanofluid delivery vehicles aim to lessen off-target effects and improve drug efficacy. In the future, nanomaterials will likely play a key role in developing personalized medicine given their great customizability, allowing drug cargo and targeting functionality to be swapped out as needed.
References:
- Sheikhpour, M., Arabi, M., Kasaeian, A., Rokn Rabei, A. & Taherian, Z. (2020). Role of Nanofluids in Drug Delivery and Biomedical Technology: Methods and Applications. Nanotechnology, Science and Applications. Volume 13. pp. 47–59. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7399455/
- Ali, A.R.I. & Salam, B. (2020). A review on nanofluid: preparation, stability, thermophysical properties, heat transfer characteristics and application. SN Applied Sciences. 2 (10). https://link.springer.com/article/10.1007/s42452-020-03427-1
- Wu, W., He, Q. & Jiang, C. (2008). Magnetic Iron Oxide Nanoparticles: Synthesis and Surface Functionalization Strategies. Nanoscale Research Letters. [Online]. 3 (11). pp. 397–415. Available from: https://dx.doi.org/10.1007/s11671-008-9174-9.. https://nanoscalereslett.springeropen.com/articles/10.1007/s11671-008-9174-9
Further Reading
- All Biomedicine Content
- Insight into Reproductive Biomedicine
- What is Nephrotoxicity?
- What is Computational Biomedicine?
- Bioinspired Materials in Biomedicine
Last Updated: Jan 31, 2023

Written by
Michael Greenwood
Michael graduated from Manchester Metropolitan University with a B.Sc. in Chemistry in 2014, where he majored in organic, inorganic, physical and analytical chemistry. He is currently completing a Ph.D. on the design and production of gold nanoparticles able to act as multimodal anticancer agents, being both drug delivery platforms and radiation dose enhancers.
Source: Read Full Article
