Parkinson’s disease (PD) is the second most common neurodegenerative disease, with patient numbers being expected to double worldwide in the next 20 years. The detailed molecular and cellular mechanisms underlying its pathogenesis remains unclear, although recent evidence has pointed towards the role of mitochondrial dysfunction in the onset of the disease. Mitochondria—small cellular ‘subunits’ involved in cell metabolism and energy generation—constantly and dynamically interact with each other, forming perpetually changing networks known as mitochondria interaction networks (MINs). The researchers therefore sought to understand the correlation between the mitochondrial impairments observed in PD and any specific network topological changes in MINs, with the aim of advancing the early diagnosis and classification of PD patients.
“Since conventional analysis focusing on individual mitochondria has not provided satisfying insights into PD pathogenesis, our pioneering work has gone a step forward by investigating the interaction networks between these organelles”, explains Dr. Feng He, Group Leader of the Immune Systems Biology Group of the LIH Department of Infection and Immunity and corresponding author of the publication.
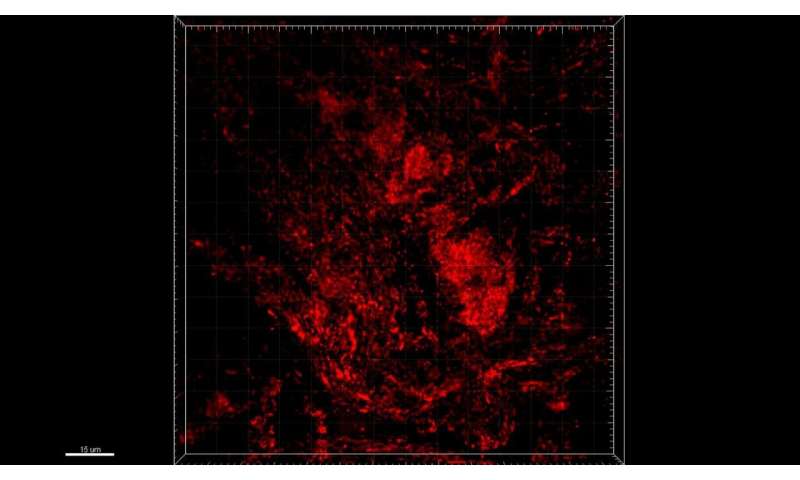
Leveraging their strong expertise in network analysis and machine learning, the scientists analyzed a large 700 Gigabyte dataset of three-dimensional mitochondrial images of colonic neurons, collected from PD patients and healthy controls, and dopaminergic neurons, derived from stem cells. They found that particular network structure features within MINs were altered in PD patients compared to controls. For instance, in PD patients, mitochondria formed connected subnetworks that were generally larger than in healthy individuals. In line with this result, the efficiency of the energy and information transmission and distribution among the different mitochondria in PD patient MINs was significantly lower than in controls, suggesting that the longer ‘transmission delays’ were associated with the larger diameter of the components of the MINs observed in PD subjects. “These different topological patterns in MINs may mean that energy and information are possibly produced, shared and distributed less competently in the neuronal mitochondria of PD patients relative to healthy controls, suggesting their connection to mitochondrial damage, deficiencies and fragmentation typical of neurodegenerative disorders”, adds Dr. He.
Moreover, the research team found these different MIN patterns to be highly correlated with the commonly-used PD clinical scores of individual patients, i.e. the Unified Parkinson’s Disease Rating Scale (UPDRS). Indeed, when applying a machine learning approach to analyze these MIN characteristics, the researchers observed that the use of a combination of those network features alone allowed them to accurately distinguish between PD patients and healthy controls.
“Our findings bring forward the potential of using particular mitochondrial network features as novel biomarkers for the early diagnosis and classification of PD patients, which might help develop a new health index. As a next step, we will explore how our results may offer new perspectives for the understanding of various other neurodegenerative diseases characterized by mitochondrial dysregulation, such as Huntington disease and Alzheimer’s, making our work a true instance of translational and transversal research”, states Prof Rejko Krüger, Director of Transversal Translational Medicine at LIH and contributing author of the study.
“This publication also constitutes a major step forward in the application of advanced machine-learning techniques to unravel the complex network interactions of cellular organelles for disease stratification. Indeed, data analytics and innovative digital technologies are a core priority area for our department and for LIH as a whole”, concludes Prof Markus Ollert, Director of the Department of Infection and Immunity and contributing author of the paper.
Source: Read Full Article
