The development of vaccines against the coronavirus disease 2019 (COVID-19) was considered the only way of reducing morbidity and mortality caused by infection with the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Currently, four vaccines against COVID-19 are being used in the European Union, of which include two messenger ribonucleic acid (mRNA)-based (BNT162b2 Pfizer-BioNTech and mRNA-1273 Moderna) and two adenovirus vector-based (ChAdOx1 nCoV-19 Astrazeneca and Ad26.COV2.S Janssen).
All vaccines are administered in a homologous prime-boost regimen with different time intervals, except Ad26.COV2.S, which is administered as a single dose. Ad26.COV2.S was found to be advantageous in deployment due to its single-dose administration, as well as favorable storage conditions.
 Study: Immunogenicity and reactogenicity of booster vaccinations after Ad26.COV2.S priming. Image Credit: Who is Danny / Shutterstock.com
Study: Immunogenicity and reactogenicity of booster vaccinations after Ad26.COV2.S priming. Image Credit: Who is Danny / Shutterstock.com
An overview of the Ad26.CoV2.S vaccine
Furthermore, both humoral and cellular immune responses are effectively induced after Ad26.COV2.S vaccination and were found to persist for eight months after vaccination. However, the vaccine efficacy (VE) of mRNA-based vaccines was found to be higher than Ad26.COV2.S. This raised concerns about whether booster vaccinations might be required to protect against future SARS-CoV-2 variants of concern (VOC).
Recent studies on homologous Ad26.COV2.S booster vaccination at 56 or 180 days after the first dose of vaccination indicated that booster vaccination led to an increase in binding antibody levels but had no impact on the T-cell immunity.
Several studies have shown that the 'mixing and matching' of the different COVID-19 vaccines has led to a broader immune response, as well as enhanced the flexibility of vaccination campaigns. Heterologous vaccination with the ChAdOx1 nCoV-19 and BNT162b2 vaccines was found to be safe and capable of eliciting a superior immune response.
A new study published on the preprint server medRxiv* compares healthcare workers (HCW) who underwent homologous Ad26.COV2.S booster vaccination to those who underwent heterologous boosting with mRNA-based vaccines BNT162b2 or mRNA-1273. The study involved a complete assessment of the participants’ immunological profile, along with a measurement of neutralizing antibodies and SARS-CoV-2-specific T-cell responses.
About the study
The current study was a single-(participant)-blinded, multi-center, randomized controlled trial that involved 434 healthy HCWs. The age of the recruited HCWs was between 18 and 65 years. Notably, none of the HCWs in this study had any severe comorbidities nor any known history of SARS-CoV-2 infection.
All recruited participants were vaccinated with Ad26.COV2.S three months before the study and were divided into four groups: Ad26.COV2.S/no boost, Ad26.COV2.S/Ad26.COV2.S, Ad26.COV2.S/mRNA1273, or Ad26.COV2.S/BNT162b2. The recruitment of participants into each group took place in a randomized manner.
All participants who were initially vaccinated with Ad26.COV2.S received a booster vaccination on day 1 of the study, while the Ad26.COV2.S/no boost group did not receive any placebo injection. Blood samples of the participants were collected on day 1 and day 28.
The study involved safety assessments to monitor any local or systemic reactions faced by the participants both after vaccination with Ad26.COV2.S and booster vaccination. Safety monitoring took place on day 1 and day 28 of the study. Humoral and cellular immunity was analyzed by assessing the presence of SARS-CoV-2 specific antibodies and SARS-CoV-2 specific T-cell responses.
Study findings
The results of the study indicated that single-dose vaccination with Ad26.COV2.S led to an increase in SARS-CoV-2 specific binding antibodies, neutralizing antibodies, as well as T-cell responses. However, the responses were superior in the case of booster vaccinations, especially heterologous boosting with mRNA-based COVID-19 vaccines.
The performance of the mRNA-1273 vaccine was better as compared to BTN162b2 in terms of its ability to induce the generation of higher binding antibodies and SARS-CoV-2 specific T-cells.
The study also indicated that 71% of HCWs already showed the presence of neutralizing antibodies after a single dose of Ad26.COV2.S. However, the response rate increased to 95.2% after homologous boosting and 100% after heterologous boosting with mRNA vaccines.
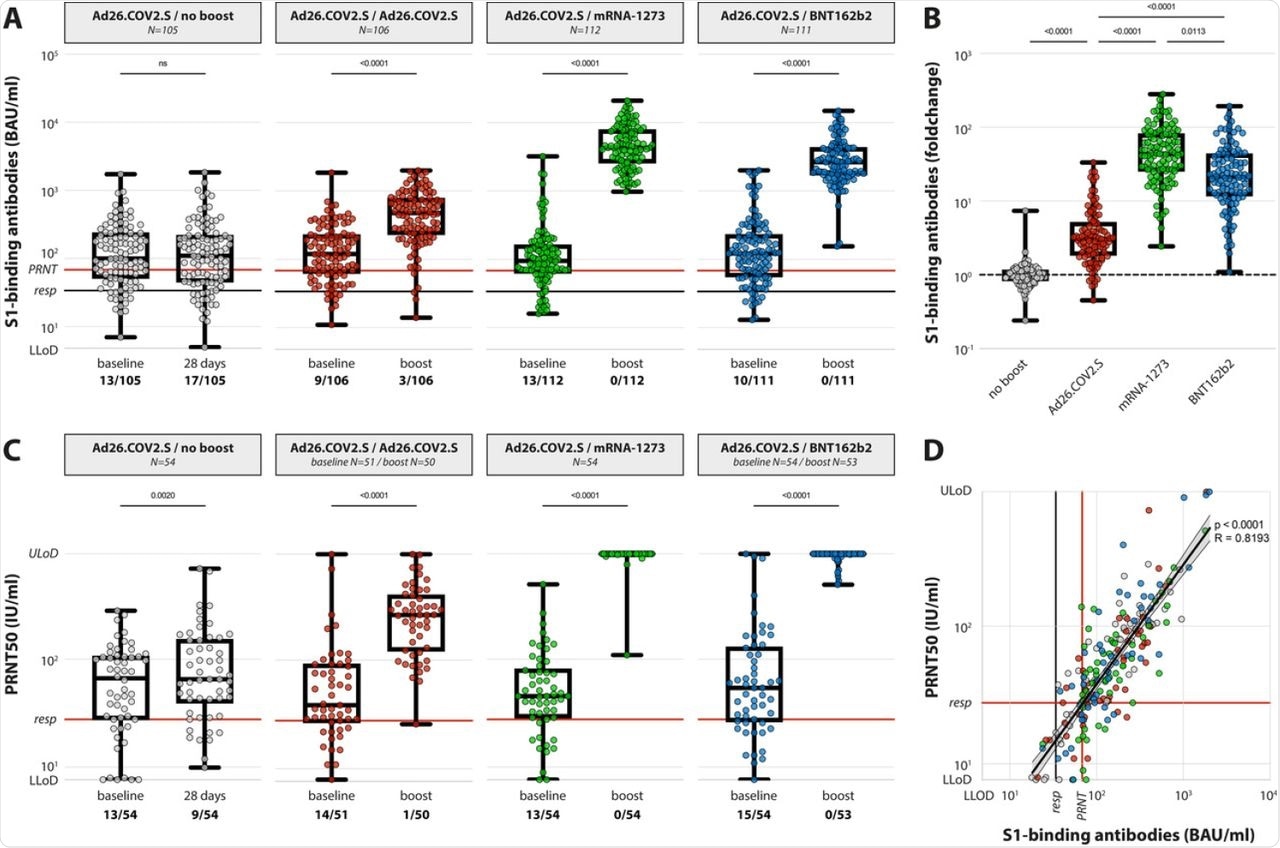 (A) Levels of binding S-specific antibodies at baseline and post booster vaccination in the 4 different groups. LLoD is 4.81 BAU/ml, responder [resp] cut-off is 33.8 BAU/ml (black line), levels >68.3 BAU/ml correlate to neutralizing capacity (red line, based on panel D). Data is presented as min-to-max box plots with individual values, timepoints were compared by Wilcoxon test. Number of participants below responder cut-off is indicated. (B) Individual fold changes were calculated by dividing the post-boost response by the pre-boost response. Data is presented as min-to-max box plots with individual values, groups were compared by Kruskal-Wallis test. (C) Levels of neutralizing antibodies at baseline and post booster vaccination in the 4 different groups. LLoD is 7.7 IU/ml, responder cut-off is set at 28.6 IU/ml. Data is presented as min-to-max box plots with individual values, timepoints were compared by Wilcoxon test. Number of participants below responder cut-off is indicated. (D) Correlation between binding S-specific antibodies and neutralizing antibodies. Spearman R = 0.83, p <0.001. Linear regression on log-transformed data was performed (Y=2.89*X-0.498), indicating that presence of neutralizing antibodies (>28.6) correlates with a binding antibody level >68.3 (red lines, resp).
(A) Levels of binding S-specific antibodies at baseline and post booster vaccination in the 4 different groups. LLoD is 4.81 BAU/ml, responder [resp] cut-off is 33.8 BAU/ml (black line), levels >68.3 BAU/ml correlate to neutralizing capacity (red line, based on panel D). Data is presented as min-to-max box plots with individual values, timepoints were compared by Wilcoxon test. Number of participants below responder cut-off is indicated. (B) Individual fold changes were calculated by dividing the post-boost response by the pre-boost response. Data is presented as min-to-max box plots with individual values, groups were compared by Kruskal-Wallis test. (C) Levels of neutralizing antibodies at baseline and post booster vaccination in the 4 different groups. LLoD is 7.7 IU/ml, responder cut-off is set at 28.6 IU/ml. Data is presented as min-to-max box plots with individual values, timepoints were compared by Wilcoxon test. Number of participants below responder cut-off is indicated. (D) Correlation between binding S-specific antibodies and neutralizing antibodies. Spearman R = 0.83, p <0.001. Linear regression on log-transformed data was performed (Y=2.89*X-0.498), indicating that presence of neutralizing antibodies (>28.6) correlates with a binding antibody level >68.3 (red lines, resp).
Similar results were found with the T-cell responses, wherein 65.8% of HCWs showed detectable SARS-CoV-2 specific T-cells after the single dose of Ad26.COV2.S. However, the response rate was found to be 100% after boosting with mRNA vaccines.
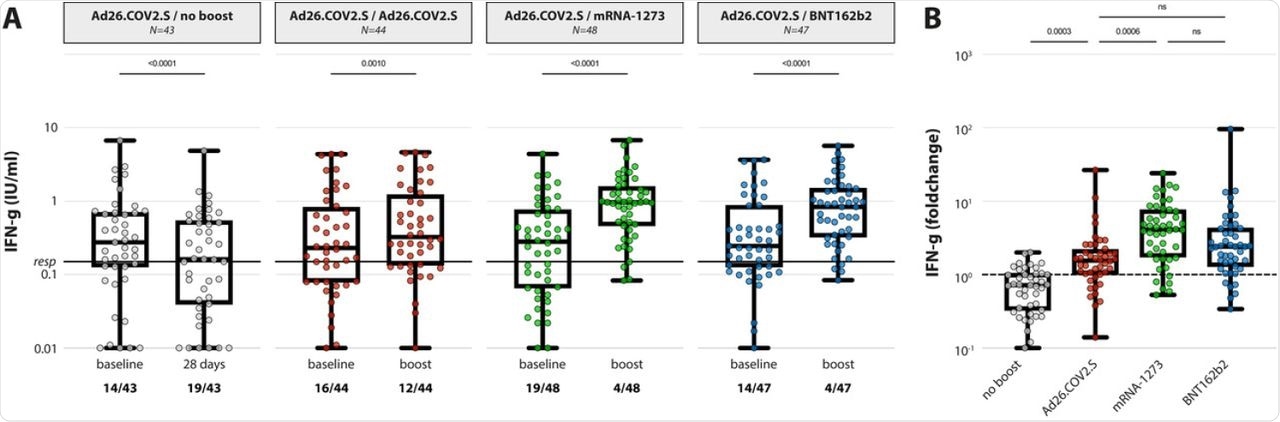 (A) IFN-y levels in plasma after stimulation of whole blood with an overlapping S pool (Ag2, Qiagen) at baseline and post booster vaccination in the 4 different groups. LLoD is 0.01 IU/ml, responder cut-off is 0.15 IU/ml. Data is presented as min-to-max box plots with individual values, timepoints were compared by Wilcoxon test. Number of participants below responder cut-off is indicated. (B) Individual fold changes were calculated by dividing the post-boost response by the pre-boost response. Data is presented as min-to-max box plots with individual values, groups were compared by Kruskal-Wallis test.
(A) IFN-y levels in plasma after stimulation of whole blood with an overlapping S pool (Ag2, Qiagen) at baseline and post booster vaccination in the 4 different groups. LLoD is 0.01 IU/ml, responder cut-off is 0.15 IU/ml. Data is presented as min-to-max box plots with individual values, timepoints were compared by Wilcoxon test. Number of participants below responder cut-off is indicated. (B) Individual fold changes were calculated by dividing the post-boost response by the pre-boost response. Data is presented as min-to-max box plots with individual values, groups were compared by Kruskal-Wallis test.
Furthermore, since the heterologous ‘mixing and matching’ of the approved vaccines were not evaluated in Phase II clinical trials, the reactogenicity of these vaccines was strictly evaluated. The reactogenicity data detected mild local and systemic reactions with only mRNA-1273 heterologous booster vaccination. However, no adverse events were reported that required hospitalization and the symptoms generally subsided within 48 hours. This suggested that both homologous and heterologous boosting was well-tolerated.
Although the study was quite effective in determining the importance of booster vaccination, it had certain limitations. The sample size of the study was quite small and only younger HCWs who did not have any comorbidities were included in the current study. Therefore, it is difficult to correlate the results of the study to the entire general population. Finally, the interval between the boosting was taken to be three months in the study, but the optimal interval still needs to be investigated.
*Important notice
medRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
- Sablerolles, R. S. G., Rietdijk, W. J. R., Goorhuis, A., et al. (2021). Immunogenicity and reactogenicity of booster vaccinations after Ad26.COV2.S priming. medRxiv. doi:10.1101/2021.10.18.21264979. https://www.medrxiv.org/content/10.1101/2021.10.18.21264979v1
Posted in: Medical Research News | Disease/Infection News | Pharmaceutical News
Tags: Adenovirus, Antibodies, Antibody, Blood, Cell, Coronavirus, Coronavirus Disease COVID-19, Efficacy, Healthcare, Homologous, Immune Response, immunity, Mortality, Placebo, Respiratory, Ribonucleic Acid, SARS, SARS-CoV-2, Severe Acute Respiratory, Severe Acute Respiratory Syndrome, Syndrome, T-Cell, Vaccine

Written by
Suchandrima Bhowmik
Suchandrima has a Bachelor of Science (B.Sc.) degree in Microbiology and a Master of Science (M.Sc.) degree in Microbiology from the University of Calcutta, India. The study of health and diseases was always very important to her. In addition to Microbiology, she also gained extensive knowledge in Biochemistry, Immunology, Medical Microbiology, Metabolism, and Biotechnology as part of her master's degree.
Source: Read Full Article
