“Closing the loop” has become one of the jargony cliches of the business world. But in the world of cancer immunotherapy, closing the loop could be an innovation that unlocks powerful therapies for hard-to-treat brain cancers called glioblastomas.
Researchers at Georgia Tech and Emory University have developed a system that uses ultrasound-induced microbubbles to help a powerful immunotherapy target brain tumors and a custom algorithm to continuously fine tune the bubbles for maximum impact.
Their closed-loop controlled focused ultrasound system proved effective in boosting survival rates in mouse models, including eradicating the entire tumor in at least one case. They described their approach Nov. 18 in the journal Science Advances.
“With closed loop focused ultrasound, we observed statistically significant improved anti-PD-1 delivery, which is the antibody that we use for our immunotherapy. We also have observed improved efficacy of the combined treatment in a survival study,” said Hohyun “Henry” Lee, a Ph.D. student in the George W. Woodruff School of Mechanical Engineering and first author of the paper.
“Recent studies have shown that focused ultrasound combined with microbubbles can enhance this immunotherapy technology. We developed an algorithm that controls the focused ultrasound to maximize the combined effect of these two technologies.”
PD-1 is a key protein on the immune system’s T cells that serves as an off-switch, preventing T cells from attacking normal, healthy cells by mistake. However, sometimes PD-1 stops T cells from targeting cancer cells. Left unbothered by the body’s immune system, those cells then can proliferate.
Anti-PD-1 drugs block the protein from shutting down T cells, freeing them to attack tumors. They’ve become a powerful weapon, particularly against melanoma and some lung cancers, but their effectiveness against brain tumors has been disappointing—partly because delivering drugs through the blood-brain barrier is a major challenge.
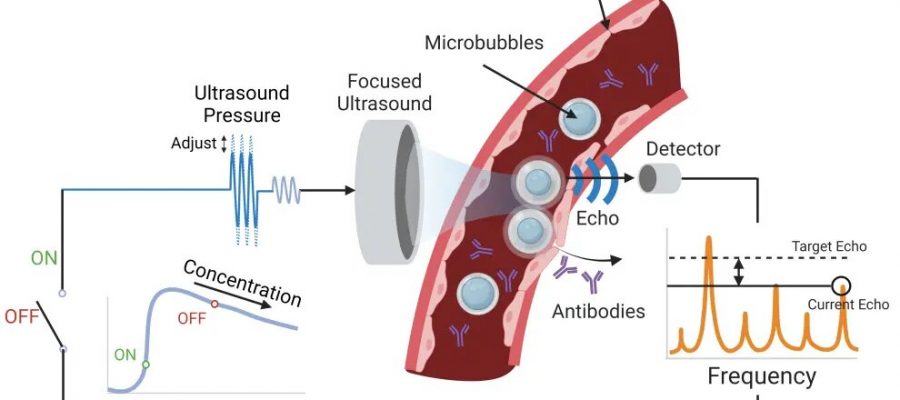
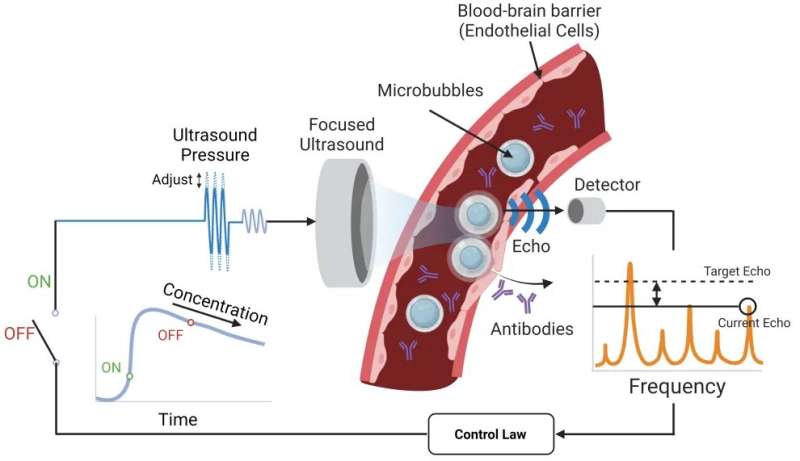
The system Lee developed with Associate Professor Costas Arvanitis is designed to get the anti-PD-1 therapy across the blood-brain barrier and into the tumor microenvironment using tiny bubbles one-thousandth of a millimeter in diameter. The focused ultrasound causes the bubbles to oscillate, which forces open the blood-brain barrier so the therapeutic agent can reach the tumor.
The researchers’ key innovation in this study is an algorithm that constantly measures the echoes of the bubbles to maintain optimum force while tracking the bubble concentration and not damaging blood vessels. That’s the closed loop: The algorithm takes in a constant flow of data about the microbubbles and adjusts accordingly. Other microbubble systems lack this level of control.
“We need to really tune this pressure or force that we apply to these bubbles with ultrasound at a very, very high level of precision, and that’s what Henry did,” said Arvanitis, who is jointly appointed in the Woodruff School and the Wallace H. Coulter Department of Biomedical Engineering. “If we have a lower vibration, we will not have the desired effect. If we have a higher level, we might create damage. It’s really about tuning these micro- to nanoscale changes in the bubble radius and doing it completely noninvasively.”
Researchers created a focused ultrasound system that uses microbubbles to open a pathway for an anti-PD-1 immunotherapy drug to reach brain tumors. They designed an algorithm that takes in a constant flow of data about the microbubbles and adjusts accordingly for optimal impact. Other microbubble systems lack this level of control.
The adaptiveness of the team’s system results in a greater safety profile and the ability to fine-tune therapies for any potential patient, Arvanitis said. The algorithm was able to detect signals that indicated trouble and adjust, sustaining just-right microbubble oscillations in a dynamic environment where the line between stability and violent bubble collapse is whisper thin.
“Closing the loop and tracking the bubble kinetics in real time is critical for this research and moving to the clinic, where every time you have a new patient and a new case,” Arvanitis said. “When we talk to our colleagues in the clinic, they tell us that you have so many constraints, and you really need to be in position to make quick decisions without compromising safety and efficacy. That’s what this controller would be able to offer.”
Other contributors to the paper included Woodruff School Professor F. Levent Degertekin, Postdoctoral Fellow Yutong Guo, Postdoctoral Fellow James L. Ross in the Emory Department of Microbiology and Immunology, and Scott Schoen from Massachusetts General Hospital.
Arvanitis said though they focused on developing the algorithm-controlled approach for brain cancer immunotherapy, it holds promise for enhancing delivery of therapeutics for other kinds of brain diseases too, like Alzheimer’s or Parkinson’s.
“Because it’s so safe, it gives you the opportunity to treat multiple times and do treatment over a longer period. That could be key for neurodegenerative diseases,” he said.
For now, the team has demonstrated the system in small animal models, but they said their approach is easily scaled up to work with existing clinical systems. They hope to work with collaborators to include their approach in ongoing early clinical trials where they could further test safety and efficacy for human patients.
“We will continue advancing the method and instrumentation in the coming years to make this approach more effective and potentially more broadly applicable,” Degertekin said.
More information:
Hohyun Lee et al, Spatially targeted brain cancer immunotherapy with closed-loop controlled focused ultrasound and immune checkpoint blockade, Science Advances (2022). DOI: 10.1126/sciadv.add2288
Journal information:
Science Advances
Source: Read Full Article