Since November 2021, the SARS-CoV-2 Omicron has spread expeditiously all over the world and gradually superseded the previous attention-capturing the SARS-CoV-2 variants of concern (VOCs). The Omicron variant harbors more than 30 mutations in the spike protein, leading to immune evasion from many therapeutic neutralizing antibodies. Therefore, it is of immense clinical significance to develop a potent neutralizing antibody against the Omicron variant.
On March 28, 2022, Prof. Zhu Yongqun at the Zhejiang University Life Sciences Institute, in collaboration with Prof. Deng Kai at Sun Yat-sen University, Prof. Chen Zhiwei at the University of Hong Kong, and Prof. Ye Lilin at Third Military Medical University, published a paper entitled “35B5 antibody potently neutralizes SARS-CoV-2 Omicron by disrupting the N-glycan switch via a conserved Spike epitope” in the journal Cell Host & Microbe.
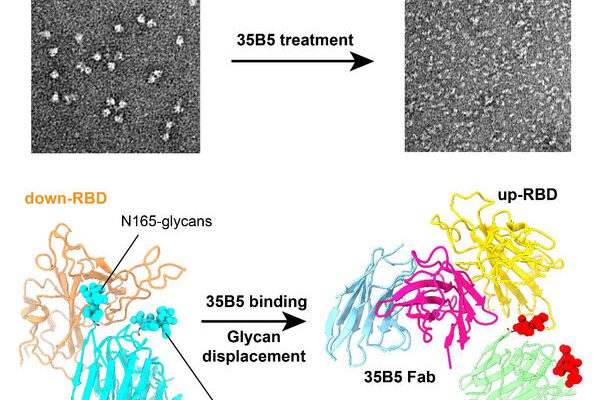
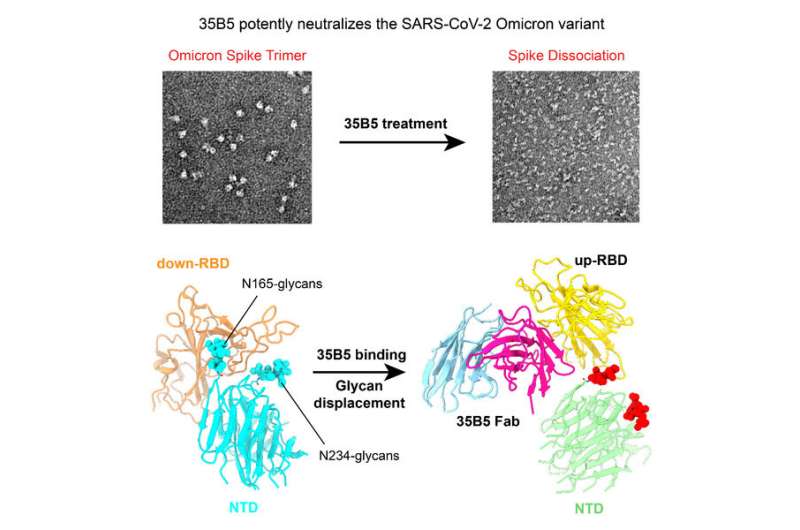
Zhu Yongqun et al. discovered that 35B5, a receptor-binding domain (RBD)-targeting monoclonal antibody, exhibited potent nanomolar neutralizing efficacy to Omicron. To investigate the structural basis for immune evasion and 35B5, they employed a cryo-electron microscopy (EM) single-particle method to determine the complex structure of the Omicron S ectodomain (Omicron S-ECD) trimer with 35B5 Fab. They found that Omicron spike was featured by tight trimeric packing and high thermostability, as well as significant antigenic shifts and structural changes, within the RBD, N-terminal domain (NTD), and subdomains 1 and 2.
However, these changes did not affect the targeting of the invariant 35B5 epitope. 35B5 could potently neutralize SARS-CoV-2 Omicron and other variants by inducing significant conformational changes within a conserved N-glycan switch that controls the transition of RBD from the “down” state to the “up” state, which allows for the recognition of the host entry receptor ACE2.
Source: Read Full Article