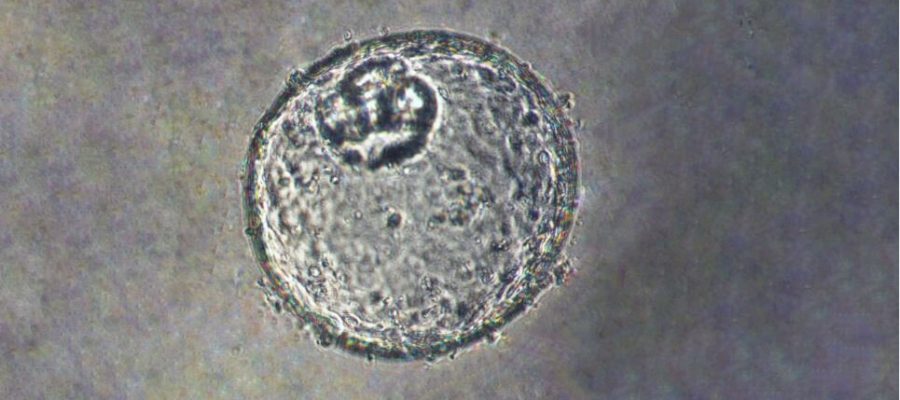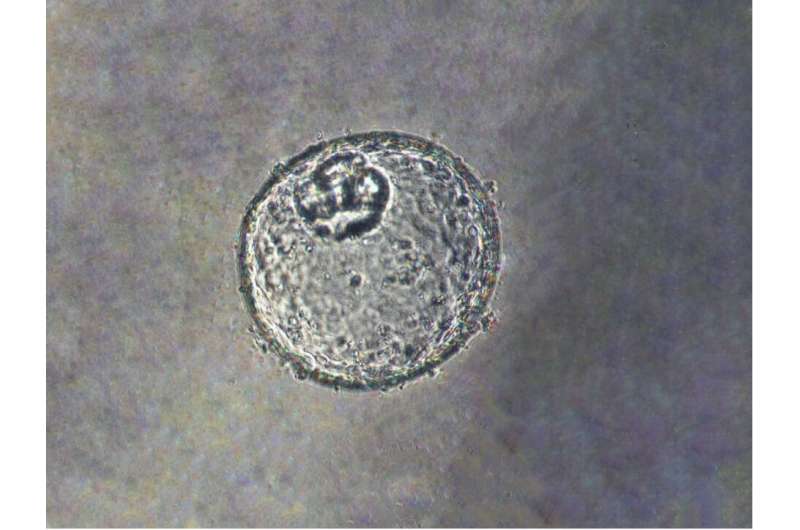
Researchers at Johns Hopkins Medicine say they have created a laboratory-grown three-dimensional “organoid” model that is derived from human tissue and designed to advance understanding about how early stages of cancer develop at the gastroesophageal junction (GEJ)—the point where the digestive system’s food tube meets the stomach.
A report on the organoid model findings, published in Science Translational Medicine, also reveals a possible biological target for treating GEJ cancers with a drug that the researchers have already shown can slow down or stop growth of such tumors in mice.
According to the American Cancer Society, gastroesophageal cancers claim more than a million lives every year worldwide, with rates of GEJ cancer increasing more than twofold in recent decades, from 500,000 to 1 million new cases annually. Acid reflux, smoking and Helicobacter pylori bacterial infection of the stomach are well-established risk factors for tumors of the esophagus and stomach.
But experts say it’s been difficult to show how cancer begins at the junction of the stomach and the esophagus, in part due to a lack of biologically relevant GEJ-specific early disease models for research.
“Because we don’t have a unique model that distinguishes GEJ tumors, gastroesophageal cancers often are classified as either esophageal cancer or gastric cancer—not GEJ cancer,” says gastroenterologist Stephen Meltzer, M.D., the Harry and Betty Myerberg/Thomas R. Hendrix and American Cancer Society Clinical Research Professor of Medicine at the Johns Hopkins University School of Medicine and corresponding author of the study.
“Our model not only helps identify crucial changes happening during tumor growth at the GEJ, but also establishes a strategy for future studies to help understand tumors of other organs.”
Meltzer and a team of experts in cell biology, epigenomics, lipid profiling and big data analysis created the GEJ disease model by taking normal human biopsy tissue from patients receiving upper endoscopies. Organoids comprise three-dimensional collections of cells derived from stem cells that can replicate characteristics of an organ or what an organ does, such as making specific kinds of cells.
Using clustered regularly interspaced palindromic repeats (CRISPR/Cas9), a gene editing technology, the researchers then knocked out two key tumor suppressor genes (TP53 and CDKN2A) in the organoids. Dual knockout of these genes caused cells to become more cancerous, with more rapid growth and microscopic features closer to malignancy. These altered organoids also formed tumors in immunodeficient mice.
The team further found abnormalities in a class of molecules (lipids) that store energy but also exert a variety of other functions, and identified platelet activating factor as a key upregulated lipid in GEJ organoids. Platelets circulate in the bloodstream and bind together or clot when they recognize damaged blood vessels, and they can cause clotting diseases in some people.
Researchers used WEB2086, which stopped the growth of implanted GEJ organoid tumors. WEB2086, a compound approved by the Food and Drug Administration and used to treat platelet diseases, inhibits platelet activating factor receptors in mine.
Meltzer says more preclinical studies may be needed before using the compound for human patients, but that organoids may help advance such studies.
“Combining organoids with this gene editing method [CRISPR/Cas9] is a potentially fruitful strategy for studying other human tumors in general,” says Meltzer.
More information:
Hua Zhao et al, Generation and multiomic profiling of a TP53/CDKN2A double-knockout gastroesophageal junction organoid model, Science Translational Medicine (2022). DOI: 10.1126/scitranslmed.abq6146
Journal information:
Science Translational Medicine
Source: Read Full Article