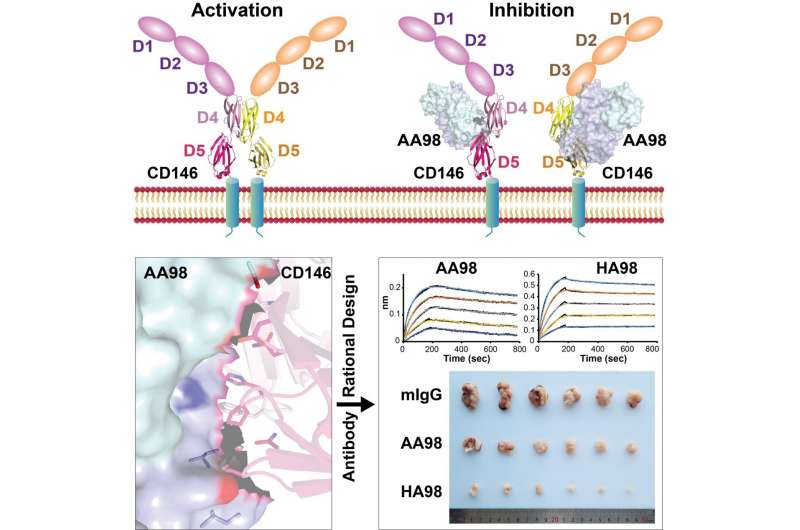
Recently, Prof. Xie Can from the High Magnetic Field Laboratory of the Hefei Institutes of Physical Science (HFIPS), in a collaboration with Prof. Yan Xiyun’s lab from the Institute of Biophysics, reported the structural basis of mAb AA98’s inhibition on CD146-mediated endothelial cells (EC) activation and designed higher affinity monoclonal antibody HA98 for cancer treatment.
CD146 is an adhesion molecule that plays important roles in angiogenesis, cancer metastasis, and immune response. Prof. Yan Xiyun’s lab has been focused on the function of CD146 and the underlying mechanism, aiming to develop antibody drugs targeting CD146. Their previous studies demonstrated that CD146 triggered the signaling cascade via dimerization induced by various ligands. AA98, a monoclonal antibody (mAb) binding to CD146, shows inhibitory effects on tumor growth. However, the structural basis of CD146 activation and AA98 inhibition remain to be addressed.
In this research, the researchers described a crystal structure of the CD146/AA98 Fab complex at a resolution of 2.8 angstrom. Structural analysis elucidated AA98 stabilized CD146 in monomer conformation thus inhibited EC activation. A higher-affinity AA98 variant (named HA98) was then rationally designed based on the complex structure in this study.
Further experiments on animal models with HA98 revealed superior inhibitory effects on tumor growth to those of AA98, which suggested future applications of this antibody in cancer therapy.
Source: Read Full Article
