The spinal column consists of 24 vertebrae that provide axial support to the torso and protection to the spinal cord that runs through its central cavity. The vertebrae are connected by means of intervertebral discs. These discs are highly hydrated, flexible and highly mechanically resistant. They allow the column its flexibility and act as shock absorbers during daily activities such as walking, running and in impact situations, such as jumping.
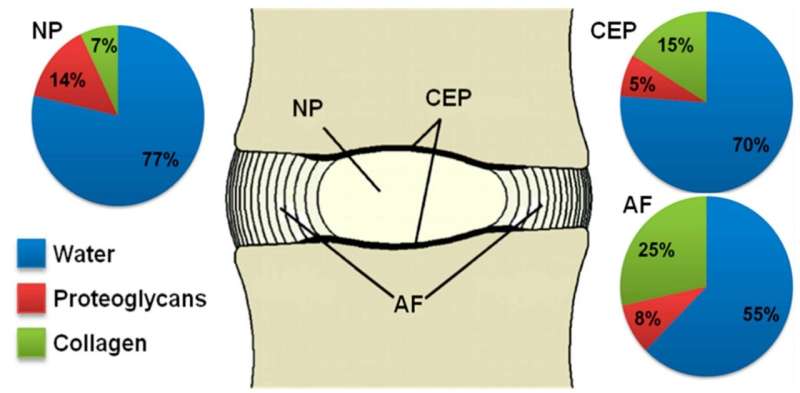
These unique features are made possible by the discs’ tissue composition and structure. At its center, there is a gel-like substance called nucleus pulposus (NP). This is surrounded by a fibrocartilage, the annulus fibrosus (AF), that laterally confines NP and enables high fluid pressure within, thus stabilizing the disc mechanically, like an inflated tyre. Upwards and downwards of the NP, are thin layers of cartilage (cartilage endplates, CEP), which separate the NP and the inner part of the AF of the vertebral bone. These layers of cartilage regulate the exchange of water and important biomolecules between the vertebrae and the NP, thus contributing to both the mechanics and the functional biological regulation of the disc.
A recent study published in the journal Bioinformatics presents a model to study the intervertebral disc resorting to experimental knowledge, and for the first time, network modeling solutions in systems biology, to mimic the cellular behavior of NP cells exposed to a 3-D multifactorial biochemical environment. This research has been carried out by members of the BCN MedTech Research Unit, a center attached to the UPF Department of Information and Communication Technologies (DTIC), with Laura Baumgartner, a UPF Ph.D. student as first author of the article, under the guidance of Jérôme Noailly, co-author of the study.
In-silico modeling to study intervertebral disc degeneration
Intervertebral disc degeneration is defined by a progressive loss of water and structure, and consequently of functionality. It differs from the natural aging of the disc by the early onset of its symptoms, which has serious effects on the workforce. It is a major risk factor for chronic lower back pain, which can in turn cause disability.
The progressive degradation of the disc consists of accumulations of microscopic lesions, most likely caused by a (prolonged) alteration of the activity of local cells, which are essential to preserve tissue integrity. The factors leading to the change in this cell activity are not fully known and seem to be caused by interactions of multiple mechanical and biochemical stimuli, which limits in vivo or in vitro exploration. This lack of knowledge means that treatments for debilitating lower back pain are not able to solve the root problem, and so focus mainly on pain control with highly variable and short-term effectiveness.
In-silico modeling is particularly attractive because it allows integrating the influence of different stimuli on cells of the nucleus pulposus exposed to a 3-D multifactorial environment. It also offers the chance to test a large number of possible scenarios of degeneration that might explain the great variability of clinical cases, which would be impossible by performing experiments.
As Laura Baumgartner explains in greater detail, “The in-silico modeling used in this study was the agent-based model, simulating a volume of 1mm³ with 4000 cells of the nucleus pulposus as agents. Cellular activity in the nucleus pulposus was estimated through the expression of messenger RNA of various components of the tissue that may be affected in the event of intervertebral degeneration: central proteoglycan proteins that form aggrecan aggregates and type I, II and III collagen, and two proteases that degrade the tissue, the enzyme stromelysin-1 (MMP-3) and ADAMTS, with a particular affinity for aggrecans.” The values were obtained for inflamed and non-inflamed cells. In addition, cell viability was estimated by means of different biochemical profiles. The regulation of this cellular activity will determine the evolution of the composition of the disc tissues and their ability to maintain their integrity, retain water and fully undertake their role.
This advanced modeling, the first results of which have been validated, offers for the first time the possibility of predicting risk factors such as metabolic problems that can affect the nutrition of the intervertebral disc cells, deregulation problems of the inflammatory system that can lead to its over-activity, or morphological or mechanical risk factors, among others.
The study spawns a new horizon in the exploration of the intervertebral disc and its translation to better managing a large number of back pain problems. This potential will be explored in great depth in the new European project Disc4All (H2020-MSCA-ITN-ETN-2020 GA: 955.735), coordinated by Jérôme Noailly, which began in late 2020 and in which 15 theses are to be conducted focusing on the integrated description of disc degeneration mechanisms and on the exploitation of these descriptions to stratify patients with the ultimate goal of finding new therapeutic targets.
Advancing the knowledge of intervertebral disc regeneration
Source: Read Full Article
