CAR-T cell therapy is a last hope for many patients with blood, bone marrow or lymph gland cancer when other treatments such as chemotherapy are unsuccessful. A limiting factor of this otherwise very effective and safe therapy is that the cells used in the process quickly reach a state of exhaustion.
Researchers at the University of Freiburg have now been able to prevent this exhaustion and thus significantly improve the effect of the therapy in a preclinical animal model. The new results have been published in the journal Nature Immunology.
CAR-T cells are one of the personalized cancer therapies and have been used in specialized centers in Europe since 2018. In this complex treatment, immune cells, or more precisely T cells, are taken from the blood of cancer patients, genetically engineered in the laboratory with a chimeric antigen receptor (CAR) and then re-administered.
The receptor helps the T cells to identify and kill cancer cells. As a result, the therapy utilizes the body’s own cells to permanently eradicate the cancer.
A simplified T-cell receptor
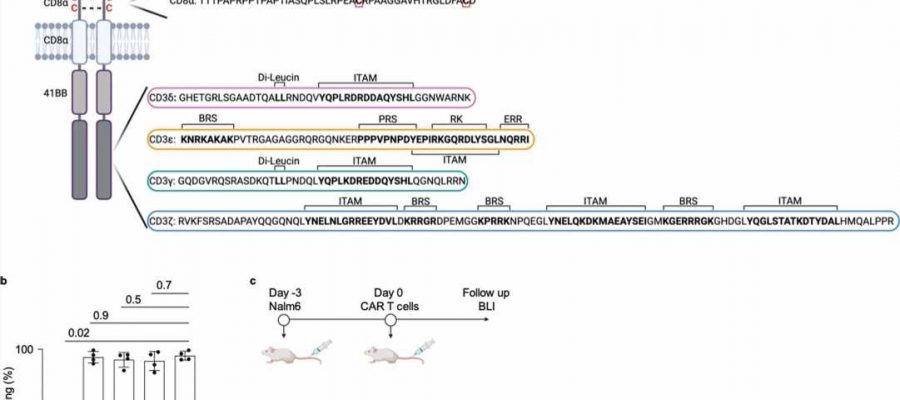
The CAR functions like a sensor with which the T cell recognizes characteristic surface features of cancer cells. The synthetic CAR consists in part of elements of the natural T cell receptor, but its structure is greatly simplified in comparison. The CAR has only one of the four different subunits that transmit the signals that trigger the activation of the immune response in unmodified T cells.
“The CARs authorized by the drug authorities all use the so-called zeta chain, which triggers a particularly strong activation of the T cell as soon as the CAR binds to the surface of a cancer cell. Whether the other three signaling chains of the T-cell receptor—gamma, delta and epsilon—can also be used for CARs has not yet been investigated,” explains Prof. Dr. Susana Minguet, who led the current study together with Prof. Dr. Wolfgang Schamel.
Both are members of the Cluster of Excellence CIBSS—Centre for Integrative Biological Signaling Studies at the University of Freiburg and are researching how the various subunits of the T cell receptor transmit signals in order to trigger an immune response.
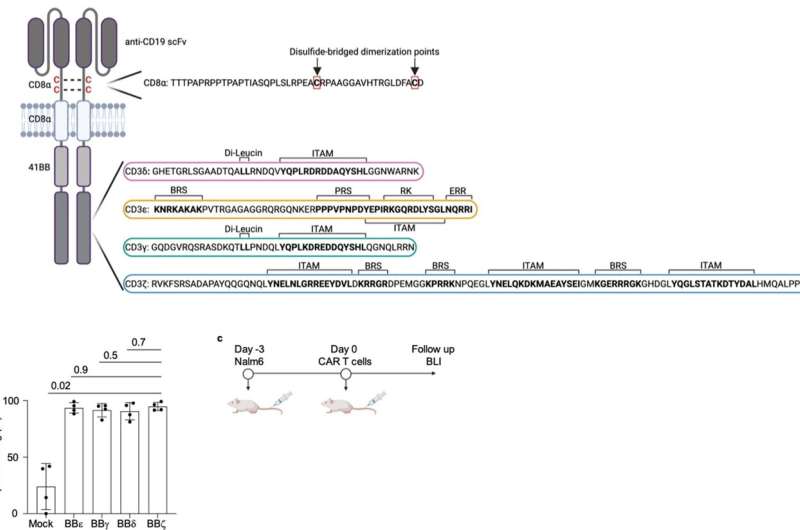
For their current study, the researchers produced four types of CAR-T cells, each expressing a CAR with each of the four signaling subunits, and tested them in a mouse model of leukemia. “Surprisingly, the zeta chain, the domain used in clinically applied CAR-T cells, showed a lower anti-tumor effect than the other three domains. These eliminated the cancer cells in the leukemia model significantly better,” explains Schamel.
Strong activation is a downside
The researchers explain the result by the fact that although the zeta chain transmits a strong activating signal to the cell, this also quickly exhausts the cell.
“It’s as if we were making the cells run an ultramarathon at maximum speed,” explains Minguet. In contrast, the delta chain, which showed the best efficacy in the current study, triggers an inhibitory signal parallel to the activation of the T cell. “This allows the immune cell to run at its optimum speed,” says Minguet.
“Our results show that CARs that use one of the other signaling domains instead of the zeta chain could mitigate or prevent the disadvantages of existing therapies with CAR-T cells,” summarizes Schamel. The researchers conclude that the development of new CAR therapies should therefore consider strategies that can achieve a more balanced immune response.
More information:
Velasco Cárdenas et al. Harnessing CD3 diversity to optimize CAR T cells, Nature Immunology (2023). DOI: 10.1038/s41590-023-01658-z. www.nature.com/articles/s41590-023-01658-z
Journal information:
Nature Immunology
Source: Read Full Article