A combination of two drugs showed promise in treating relapsed or refractory acute myeloid leukemia (AML) with mutations in the FMS-like tyrosine kinase 3 (FLT3) gene, according to a Northwestern Medicine study published in the Journal of Clinical Oncology .
The combination of venetoclax, an inhibitor of BCL2, and gilteritinib, an FLT3 inhibitor, has the potential to improve outcomes and allow patients to utilize oral medications instead of treatments that must be delivered in the hospital, according to senior author Jessica Altman, MD, professor of Medicine in the Division of Hematology and Oncology.
According to a previous study published in the New England Journal of Medicine, survival of patients with refractory or relapsed AML is improved when using gilteritinib, compared to chemotherapy. Gilteritinib targets the FLT3 mutation. Typically, relapsed or refractory AML has a median overall survival of four to seven months with standard chemotherapy approaches.
In the current phase II trial, investigators studied the efficacy of adding venetoclax to gilteritinib. Investigators treated patients with relapsed or refractory AML with venetoclax and gilteritinib daily. Of the 61 patients enrolled, 56 of them had disease with the FLT3 mutation and 64% of those with the mutation had received prior FLT3 inhibitor therapy.
The study found a molecular response, defined as FLT3-ITD VAF less than or equal to 10 in 60% of participants with FLT3 -ITD who attained a modified complete remission, which was higher than with gilteritinib alone.
Although the drug combination has shown to be more effective, Altman said, it is associated with myelosuppression. The majority of participants in the trial experienced cytopenias—a lower-than-normal blood cell count—related to this therapy, so individuals on these therapies need frequent monitoring of their blood counts. Dose interruptions and modifications of venetoclax were utilized to mitigate myelosuppression.
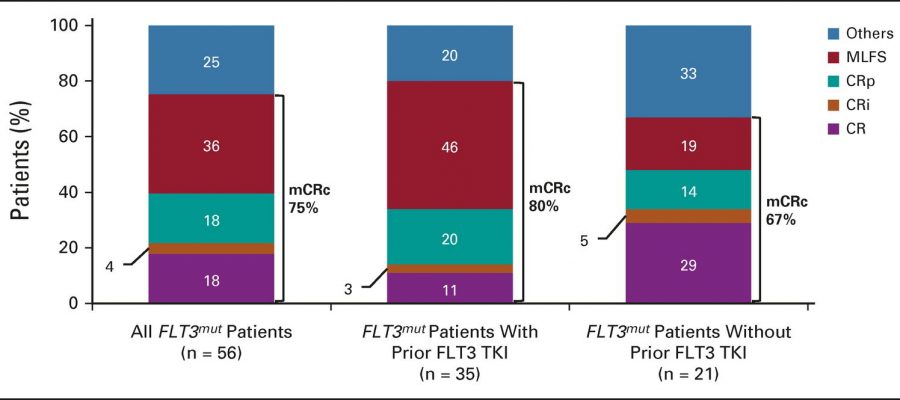
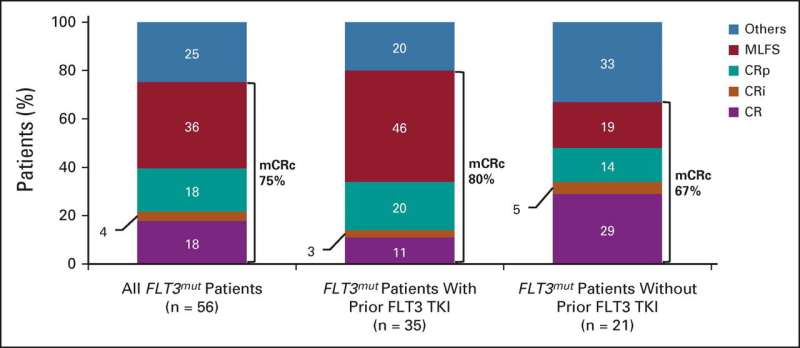
The modified composite complete response (mCRc) rate for patients with FTL3 mutations was 75%.
Prior to the approval of gilteritinib, standard of care for these patients normally required hospitalization for a month or longer with intensive chemotherapy.
“To be able to offer patients something that’s oral so that they can be at home is pretty remarkable,” Altman said. “Even if they have to come in for frequent lab work and visits with us, they still get to be comfortable in their homes.”
Source: Read Full Article