The resistance to cancer treatment is often credited to the heterogenous cellular nature within a tumor. The tumor cell-cell interaction and cell-microenvironment have a key role in the invasion and progression of tumors with important theranostics implications.
In a new study published in Scientific Reports, Nafiseh Moghimi and a team of researchers in applied mathematics at the University of Waterloo Canada, medicine and engineering at the Harvard Medical School and the Brigham and Women’s Hospital U.S., and medical genetics at the Yeditepe University Turkey, bioengineered bioprinted in vitro models of a breast cancer tumor microenvironment made of co-cultured cells mixed in a hydrogel matrix. The well-regulated architecture facilitated the development of model tumor heterogeneity.
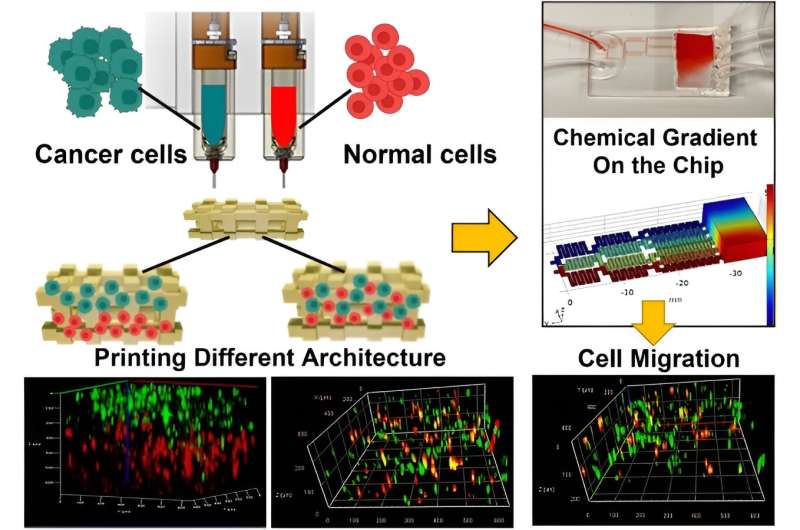
The team proposed and developed a hypothesis to represent the tumor by using a cancer cell-laden co-culture hydrogel construct. They accomplished this by modeling the microenvironment of interest in a microfluidic chip to produce a chemical gradient.
The team used breast cancer cells and non-tumorigenic mammary epithelial cells embedded in alginate-gelatin hydrogels, which they printed by using a multi-cartridge extrusion bioprinter.
The outcomes allowed for precise adjustments and cell position and arrangements in a cell co-culture system to design a variety of patient-mimetic tumor architectures. They accomplished tumor heterogeneity by randomly mixing and positioning cells in sequential layers.
Moghimi and team devised a chemical environment for chemo-attraction and constructed an excellent platform to study the behavior of cancer cells with space-time resolution.
Bioengineering cancer on a chip
Breast cancer commonly occurs in women, with more than 2 million new cases reported in 2018. The aggressiveness of the disease is owing to the heterogeneity of breast tumors leading to treatment challenges.
Since conventional models lack cellular heterogeneity of breast cancer, it is difficult to study their dependence on external stimuli, to examine their formation and study their physiology.
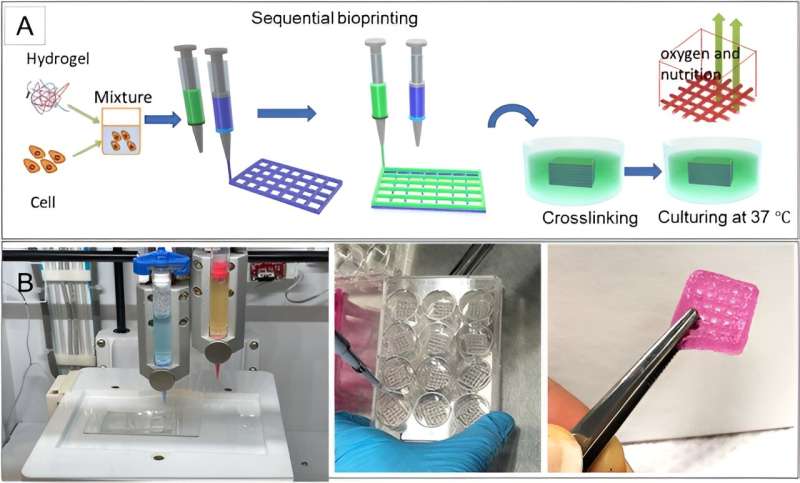
Microfluidic platforms offer an in vitro instrument to mimic physical and chemical stimuli during cell migration. Bioprinting has recently attracted much attention due to its ability to build tissue constructs by positioning cells and biomaterials precisely in a layer-by-layer procedure.
Using the method, they printed living cells and extracellular matrix components together to form a bioink that recapitulated the composition and geometry of the tumor microenvironment, while maintaining cell viability. Moghini’s research team established a platform to model phenotypic heterogeneity that occurred due to cell localization in a tumor. They accomplished this by combining 3D bioprinting approaches for in vitro tumor modeling with microfluidic devices to create model microenvironments emulating complex tumor architectures beyond conventional methods.
The mechanical properties of bioinks
During the experiments, the team prepared and printed various hydrogels with alginate and gelatin by using an INKREDIBLE bioprinter. The small nozzle diameter led to the development of a high-resolution construct but these dimensions increased the possibility of cell damage due to extremely high extrusion pressure.
The team continued bioprinting with a 22G nozzle that exerted less pressure for favorable cell viability. The concept of cell viability offers the successful fabrication of cell-printed constructs. The team conducted arrays to ensure the viability and homogeneity of live cells within the construct by applying pressure, filament condition, and quality of the printed structure based on the nozzle size and hydrogel composition.
Cell viability and 3D bioprinted co-cultures
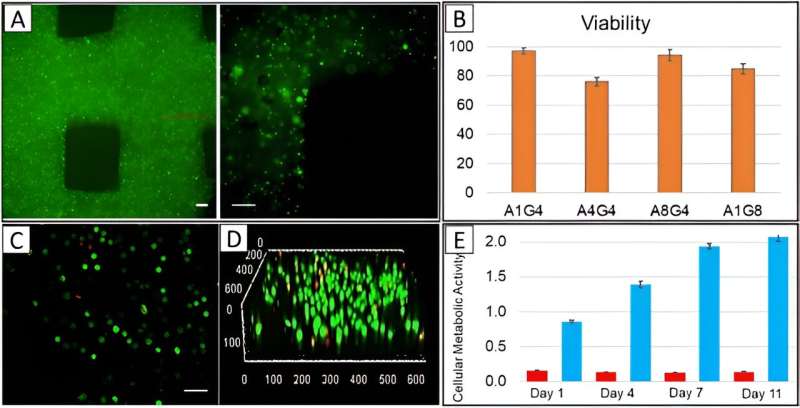
The scientists visualized the distribution of cells in the printed constructs by using cell membranes pre-stained with a green fluorescent membrane marker prior to printing, and immediately imaged them after printing with a confocal microscope. The outcomes showed the homogenous cell distribution in the construct.
The team determined the viability of diverse cells after printing them by using a live-dead staining assay, and delivered cell viability as an essential factor to successfully fabricate cell-printed constructs.
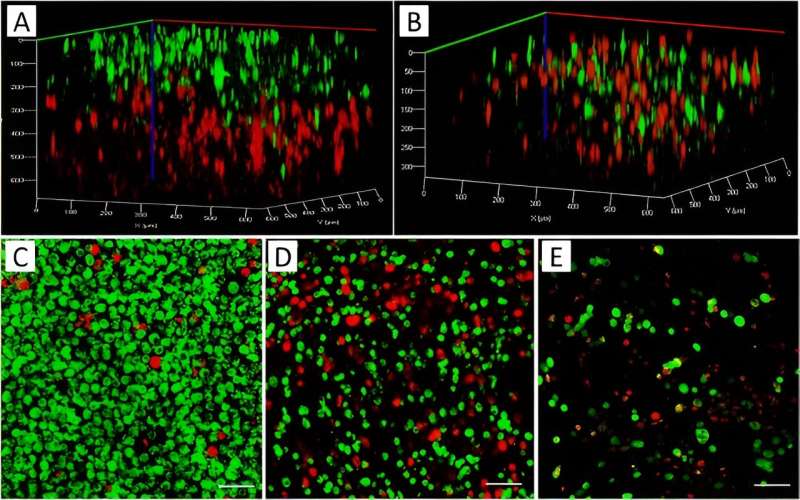
The team then combined MCF7 cancer cells of a breast adenocarcinoma cell line and MDA-MB-231, an aggressive form of triple-negative breast cancer cell line, with mesenchymal characteristics and pre-stained them with red and green biomarkers prior to printing.
At first, they prepared the two bioinks separately from each cell type and printed them sequentially. While the first layer of the printed structure contacted a red-stained cell structure, the second layer lay on top of it, stained in green. The team ensured the mixture of the two cell types and co-cultured them in a 2D chamber during a separate experiment to show how the staining procedure did not affect cell growth.
Cell growth dynamics and cell migration
The researchers studied cell growth and aggregation within bioprinted co-cultures and separately printed 3D constructs containing each cell type, and compared them with the construct containing a cell mixture of the two cell types in the bioink within a 10-day experimental timeline in tumor-like constructs and investigated cellular behavior in alginate-gelatin hydrogels.
The team used epithelial growth factor as a chemoattractant material and noted the migration of cells within co-cultures of the microfluidic device. Moghimi and colleagues used the bioprinted constructs to form diverse tumor architectures, and observed the cell migration dynamics relative to the surrounding non-cancerous cells.
Outlook
In this way, Nafiseh Moghimi and researchers exemplified tumor heterogeneity to bioengineer a tumor microenvironment on an organ chip instrument. The team implemented a tumor-on-a-chip model via 3D bioprinting of two different types of breast cancer and non-tumorigenic mammary epithelial cells to understand molecular and cellular mechanisms underlying tumor heterogeneity and treatment resistance. Such investigations can directly inform tumor diagnostics and therapeutic treatment to understand chemoresistance.
The tumor cell-cell interaction and tumor-tumor microenvironment interactions have significant influence on tumor progression and invasion. The heterogenous tumor-on-a-chip model therefore has significant impact for cancer treatment research, to build in vitro models of tumor microenvironments composed of diverse co-cultured cell types.
The team represented the tumor microenvironment on a microfluidic chip with cancer cell/non-cancerous cell hydrogel constructs to produce a chemical gradient and better understand the relationship between intratumor heterogeneity and tumor response to treatment. These outcomes will open gateways to develop new drugs for more effective personalized medicine, and provide a tumor-on-a-chip model to facilitate the understanding of cancer behavior.
More information:
Nafiseh Moghimi et al, Controlled tumor heterogeneity in a co-culture system by 3D bio-printed tumor-on-chip model, Scientific Reports (2023). DOI: 10.1038/s41598-023-40680-x
Sean V Murphy et al, 3D bioprinting of tissues and organs, Nature Biotechnology (2014). DOI: 10.1038/nbt.2958
Journal information:
Nature Biotechnology
,
Scientific Reports
Source: Read Full Article
